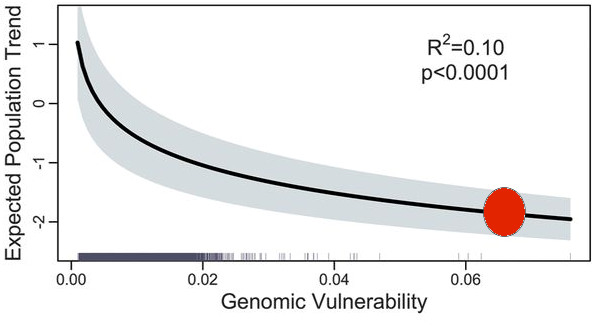
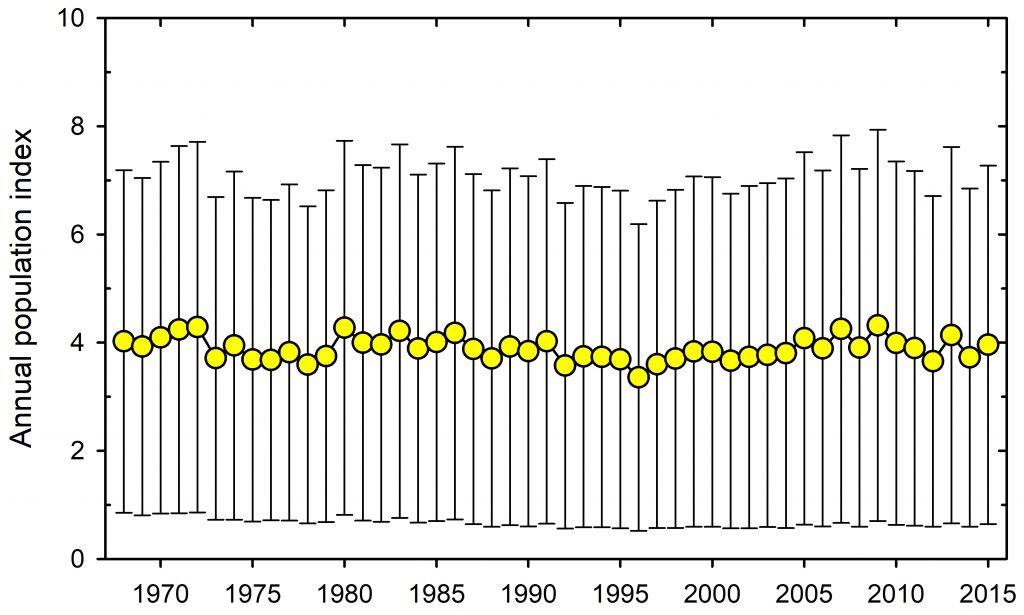
For the last 60 years a group of Stone Age scientists like myself have preached to ecology students that one needs hypotheses to do proper science. Now it has always been clear that not all ecologists followed this precept, and a recent review hammers this point home (Betts et al. 2021). I have always asked my students to read the papers from the Stone Age about scientific progress – Popper (1959), Platt (1964), Peters (1991) and even back to the Pre-Stone Age, Chamberlin (1897). There has been much said about this issue, and the recent Betts et al. (2021) paper pulls much of it together by reviewing papers from 1991 to 2015. Their conclusion is dismal if you think ecological science should make progress in gathering evidence. No change from 1990 to 2015. Multiple alternative hypotheses = 6% of papers, Mechanistic hypotheses = 25% of papers, Descriptive hypotheses = 12%, No hypotheses = 75% of papers. Why should this be after years of recommending the gold standard of multiple alternative hypotheses? Can we call ecology a science with these kinds of scores?
The simplest reason is that in the era of Big Data we do not need any hypotheses to understand populations, communities, and ecosystems. We have computers, that is enough. I think this is a rather silly view, but one would have to interview believers to find out what they view as progress from big data in the absence of hypotheses. The second excuse might be that we cannot be bothered with hypotheses until we have a complete description of life on earth, food webs, interaction webs, diets, competitors, etc. Once we achieve that we will be able to put together mechanistic hypotheses rapidly. An alternative statement of this view is that we need very much natural history to make any progress in ecology, and this is the era of descriptive natural history and that is why 75% of papers do not list the word hypothesis.
But this is all nonsense of course, and try this view on a medical scientist, a physicist, an aeronautical engineer, or a farmer. The fundamental principle of science is cause-and-effect or the simple view that we would like to see how things work and why often they do not work. Have your students read Romesburg (1981) for an easy introduction and then the much more analytical book by Pearl and Mackenzie (2018) to gain an understanding of the complexity of the simple view that there is a cause and it produces an effect. Hone et al. (2023) discuss these specific problems with respect to improving our approach to wildlife management
What can be done about the dismal situation described by Betts et al. (2021)? One useful recommendation for editors and reviewers would be to request for every submitted paper for a clear statement of the hypothesis they are testing, and hopefully for alternative hypotheses. There should be ecology journals specifically for natural history where the opposite gateway is set: no use of ‘hypothesis’ in this journal. This would not solve all the Betts et al. problems because some ecology papers are based on the experimental design of ‘do something’ and then later ‘try to invent some way to support a hypotheses’, after the fact science. One problem with this type of literature survey is, as Betts et al. recognized, is that papers could be testing hypotheses without using this exact word. So words like ‘proposition’, ‘thesis’, ‘conjectures’ could camouflage thinking about alternative explanations without the actual word ‘hypothesis’.
One other suggestion to deal with this situation might be for journal editors to disallow all papers with hypotheses that are completely untestable. This type of rejection could be instructive to authors to assist rewriting your paper to be more specific about alternative hypotheses. If you can make a clear causal set of predictions that a particular species will go extinct in 100 years, this could be described as a ‘possible future scenario’ that could be guided by some mechanisms that are specified. Or if you have a hypothesis that ‘climate change will affect species geographical ranges, you are providing a very vague inference that is difficult to test without being more specific about mechanisms, particularly if the species involved is rare.
There is a general problem with null hypotheses which state there is “no effect”. In some few cases these null hypotheses are useful but for the most part they are very weak and should indicate that you have not thought enough about alternative hypotheses.
So read Platt (1964) or at least the first page of it, the first chapter of Popper (1959), and Betts et al. (2021) paper and in your research try to avoid the dilemmas they discuss, and thus help to move our science forward lest it become a repository of ‘stamp collecting’.
Betts, M.G., Hadley, A.S., Frey, D.W., Frey, S.J.K., Gannon, D., Harris, S.H., et al. (2021) When are hypotheses useful in ecology and evolution? Ecology and Evolution, 11, 5762-5776. doi: 10.1002/ece3.7365.
Chamberlin, T.C. (1897) The method of multiple working hypotheses. Journal of Geology, 5, 837-848 (reprinted in Science 148: 754-759 in 1965). doi. 10.1126/science.148.3671.754.
Hone, J., Drake, A. & Krebs, C.J. (2023) Evaluation options for wildlife management and strengthening of causal inference BioScience, 73, 48-58.doi: 10.1093/biosci/biac105.
Pearl, J., and Mackenzie, D. 2018. The Book of Why. The New Science of Cause and Effect. Penguin, London, U.K. 432 pp. ISBN: 978-1541698963.
Peters, R.H. (1991) A Critique for Ecology. Cambridge University Press, Cambridge, England. ISBN: 0521400171.
Platt, J.R. (1964) Strong inference. Science, 146, 347-353.doi: 10.1126/science.146.3642.347.
Popper, K.R. (1959) The Logic of Scientific Discovery. Hutchinson & Co., London. ISBN: 978-041-5278-447.
Romesburg, H.C. (1981) Wildlife science: gaining reliable knowledge. Journal of Wildlife Management, 45, 293-313. doi:10.2307/3807913.