This post is meant to be a mostly comprehensive list and explanation of the non-molecular phenotypes that our lab measures on our various plant populations. At the moment, it is definitely not there yet (for one thing, it has a clear sunflower bias), so please contribute!
This post is meant to be a mostly comprehensive list and explanation of the non-molecular phenotypes that our lab measures on our various plant populations. At the moment, it is definitely not there yet (for one thing, it has a clear sunflower bias), so please contribute!
You can add links to pictures, powerpoints, methods papers, etc. If you measured a particular trait in a different way, please add another line to the table (if you’re not proficient at HTML, the easiest way, which is not very easy, is probably to modify this google excel file and then copy and paste the whole modified section into the post while deleting the older version here) and include any justifications for your particular method. The full names from the WHO column are listed under “Contributors” at the bottom of this post. If something is not totally clear, contact the person who wrote it! Additionally, there are several instructional phenotyping documents (.doc and .ppt) sent to our lab from the Burke lab in Georgia. They sometimes do things a little bit differently than we do, so it may be helpful to take a look at their documents and decide what you prefer.
Note: Some of the descriptions below reference a standardized sunflower life stage (e.g. R5.1). A pdf explaining these can be found here. These stages are mainly applicable to the domesticated sunflower, although I have successfully adapted them when phenotyping Helianthus argophyllus and wild H. annuus.
Table 1. Traits for seeds and seedlings
| TRAIT | UNITS | SYSTEM | WHO | HOW |
| inbibition | fraction | wild and domestic sunflower | HCR | proportion of scarified seeds that swell enough to visibly protrude beyond the cut end of the shell |
| germination | fraction | wild and domestic sunflower | HCR | proportion of seeds with radicle protruding at least 2mm, usually recorded for 1-3 days after scarifying |
| germination | fraction | wild and domestic sunflower | BTM | proportion of seeds to produce both a root and shoot by 7 days post-scarifying |
| seedling length | mm | wild and domestic sunflower | HCR | seedlings are photographed (usually still in a petri dish) with a scale object in the photo (in an emergency, remember that the petri dish is a defined size!). images are imported into Image J and the total seedling length (hypocotyl plus root) measured using the Freehand Lines tool. |
Table 2. Growth and size traits for adult plants (pre-harvest).
| TRAIT | UNITS | SYSTEM | WHO | HOW |
| SIZE | ||||
| plant height | cm | wild and domestic sunflower | HCR/BTM/NCK | length along stem from base to apex at X age; if flowering, length to back of terminal head |
| stem diameter | cm | wild and domestic sunflower | HCR | measured at base of stem above soil with calipers; I usually take the maximum from 3 measurements as many stems are not perfectly cylindrical |
| stem diameter | cm | wild and domestic sunflower | BTM | measured perpendicular to first leaf node nearest base of stem |
| leaf number | integer | wild and domestic sunflower | BTM | number of true leaves >2mm long at X age |
| basal leaf number | integer | Diffuse knapweed (rosette) | KGT | count leaves which emerge from root crown, at soil level. |
| bolting-stem leaf number | integer | Diffuse knapweed (rosette) | KGT | count leaves which branch from bolted stem. |
| BRANCHING | ||||
| main stem with central head | logical | domestic sunflower | UGA | yes is main stem with large head is present at R9 |
| branching | logical | domestic sunflower | UGA | yes if primary branches (any size but with terminal flower) present at R9 |
| longest branch length | cm | domestic sunflower | UGA | length of longest primary branch at R9 (any size but with terminal flower) |
| bud number | integer | domestic sunflower | UGA | number of axillary buds that do not end in terminal flower at R9 |
| branch number | integer | domestic sunflower | UGA | total number of branches (any size but with terminal flower) at R9 |
| branch number | integer | wild and domestic sunflower | HCR | total number of branches over 2mm thick |
| opposite | integer | wild and domestic sunflower | HCR | number of branches directly opposite each other in a horizontal plane (=2x number of opposite branch pairs) |
| alternate | integer | wild and domestic sunflower | HCR | number of branches with no branch directly opposite of them on the stem |
| number of nodes | integer | wild and domestic sunflower | NCK | number of leaf nodes (counting opposite nodes as 1) on primary stem at R9 |
| degree of branching | integer | wild and domestic sunflower | NCK | 0 if no branches, 1 if only primary branches, etc. at R9 |
| LEAVES | ||||
| leaf area | cm2 | wild and domestic sunflower | HCR | leaves are photographed against a white background with a scale object (usually a ruler) in the image, images are transformed into binary (black/white) in Image J, area is measured with reference to the scale object using the Wand (tracing) tool. this can also be set to provide shape descriptors at the same time. I like circularity (scaled 0-1, with 1 being a perfect circle) or roundness (minor axis/major axis; note that roundness (or aspect ratio) could be used to compare datasets that contain leaf length & width measurements but not area) |
| specific leaf area | cm2/g | wild and domestic sunflower | UGA/BTM | SLA is a physiological measure correlated to plant water use efficiency, calculated as the ratio of fresh leaf area (measured as above using a scanned image) to dry leaf mass. We used the most apical fully expanded unshaded leaf (without petiole), ideally green, healthy, free of holes, and collected in the AM. Leaves can be kept at 4C for up to four days. After scanning (with a standard resolution, e.g. 300DPI, you don’t need to include a scale object), they are dried for at least 3 days at 60C and then weighed. |
| specific leaf area | sq cm/mg | Diffuse knapweed (rosette) | KGT | harvest adult leaf, cut into pieces such that the leaf lays flat with no/minimal leaf tissue overlapping, and scan, making sure that both the label and a consistent size marker are visible. Best to do within a few hours of harvest, and one leaf per scan if possible (for batch processing in ImageJ). After scanning, place in paper bag to oven dry, then weigh. |
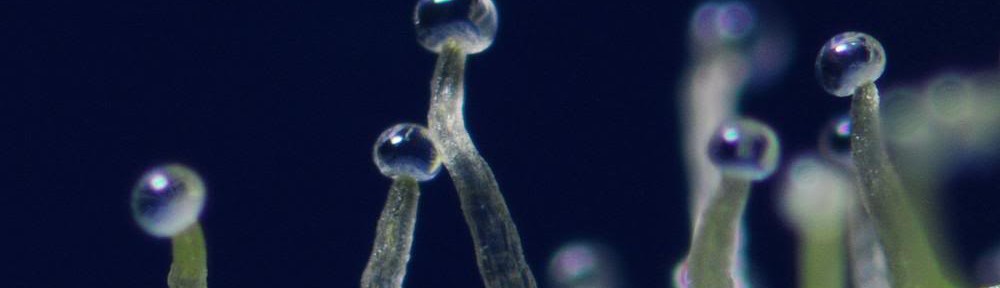
| glandular trichome density | integer per cm2 | wild and domestic sunflower | HCR | density of glandular trichomes on the abaxial leaf surface is determined by counting the number of glandular trichomes contained in a 3 mm2 ocular grid, observed at 25X magnification (set the dial to 2.5 on our dissecting scope and count the whole ocular grid), for three regions of the leaf, roughly delineated as leaf tip, leaf edge, and leaf center proximal to midvein. glandular trichomes will look like tiny balls of goo from above (as opposed to nonglandular trichomes, which will look like hairs). See featured photo. |
| FLOWERS | ||||
| bolting date | date | Diffuse knapweed (rosette) | KGT | record date of bolt emergence, when bolt reaches more than 3 cm. Bolt is identifiable by the presence of branching leaves, above soil level. |
| budding date | integer (days) | wild and domestic sunflower | BTM | days from germination to first observation of apical (or non-apical if apex is damaged/not apparent) bud at R3 stage |
| flowering date | integer (days) | wild and domestic sunflower | BTM | days from germination to first observation of apical (domestic) or any (wild) flower head with pollen and/or receptive stigmas present. For domestic, sometimes use stage R5.1 (10%) of flowers on head have pollen and/or receptive stigmas present. |
| apical dominance | logical | wild and domestic sunflower | BTM | yes if first flower head to mature is at plant apex, NA if apical meristem is damaged, no if first flower head is not apical (or no single axis is dominant) |
| head diameter | cm | wild and domestic sunflower | BTM | width at horizontal axis of apical head (or largest head if apex is damaged/not apparent) |
| disc flower color | color | wild and domestic sunflower | UGA | relatively complicated system–see UGA ppt Phenotyping_pigmentation_UGA_2010.ppt |
| disc flower color | color | Helianthus argophyllus | BTM | disc floret lobes on apical (or largest if apex is damaged/not apparent) head scored as either purple/red/yellow |
| ray flower number | integer | wild and domestic sunflower | BTM | count of ray flowers with petals longer than 2mm |
| ray flower width | mm | wild and domestic sunflower | BTM | width at widest point of a single “typical” ray floret ligule measured with calipers after laying ligule (petal) flat |
| ray flower length | mm | wild and domestic sunflower | BTM | length from point of ligule fusion to tip of a single “typical” ray floret ligule, measured with calipers after laying ligule (petal) flat |
| phyllary width | mm | wild and domestic sunflower | BTM | width at widest point of a single “typical” phyllary (flower head bract) measured with calipers |
| phyllary lenth | mm | wild and domestic sunflower | BTM | length from attachment of phyllary to head to phyllary tip of a single “typical” phyllary, measured with calipers |
| flower color | color | Diffuse knapweed (rosette) | KGT | record flower color (dark purple, pale purple, white) for newly blooming flowers. |
| head number | integer | wild and domestic sunflower | BTM | number of flowerheads when plant is at R9 stage (domestic), or has no active stigmas/pollen remaining on any heads (wild) |
| peduncle length | cm | wild and domestic sunflower | BTM | length from the back of the apical flower head (or largest flower head if apex is damaged/not apparent) to the first leaf node |
| ROOTS | ||||
| root mass | g | Diffuse knapweed (rosette) | KGT | cut stem at interface between root and shoot, wash soil from roots retaining as many roots as possible,using a seive and wash basin, oven dry in paper bags, weigh. Best if grown in sand or very sandy soil. AVOID potting soil containing vermiculite, or large pieces of bark or sticks. |
| root crown diameter | mm | Diffuse knapweed (rosette) | KGT | cut stem at interface between root and shoot, measure diameter of root crown with calipers. |
| OTHER | ||||
| drought tolerance – early wilting | date | Diffuse knapweed (rosette) | KGT | at beginning of treatment, cease all watering. Record date for first signs of wilt response (first leaf droops and feels thin and tissue paper like). |
| drought tolerance – total wilting | date | Diffuse knapweed (rosette) | KGT | at beginning of treatment, cease all watering. Record date for total wilt response (all leaves droop and feel thin and tissue paper like). |
| drought tolerance – death | date | Diffuse knapweed (rosette) | KGT | at beginning of treatment, cease all watering. Record date of death, determined by resilience of youngest, central leaf to gentle pressure (i.e. poke it). If youngest, central leaf is brittle, plant is recorded as dead. |
| flood tolerance – root death | date | Diffuse knapweed (rosette) | KGT | at beginning of treatment, submerge plants in tubs filled with water to above soil level. Change water on weekly basis. Record date of root death, when plant is easily removed from soil with gentle pressure (i.e. tug on it). |
| flood tolerance – plant death | date | Diffuse knapweed (rosette) | KGT | at beginning of treatment, submerge plants in tubs filled with water to above soil level. Change water on weekly basis. Record date of death, when all leaves of rosette are easily broken apart with gentle pressure (i.e. tug on leaves). |
| herbivore preference | % leaf disc remaining or winner/loser | Diffuse knapweed (rosette) | KGT | place into one 4 cm sq well of an appropriate tray, a moistened cotton pad topped with two leaf punches, one from each competing plant (the cotton pad keeps the leaves from drying out during the trial). Then add the herbivore (specialist weevil), placed equidistant from the two leaf discs, and leave to eat until one of the leaf discs is half consumed, or 6 hours. The plants can then be scored as winner/loser. Alternately, the leaf discs can be placed between two transparency sheets swabbed with mineral oil, scanned, and assessed for % consumed using software such as ImageJ. |
| stem hairiness | scale | Helianthus argophyllus | BTM | stem hairiness was scored on a 20 cm basal stem section on a scale from 1 – few/no hairs to 5 – stem covered in dense, wooly hairs |
| stem color | scale | Helianthus argophyllus | BTM | stem color was scored on depilated basal stem section on a scale from 1 – light green to 5 – deep purple pigmentation |
Note: Botanists love to make up anatomical terms, and Compositae botanists are no exception! Ligule, disc vs. ray flower/floret, flower head, receptacle, phyllary, and peduncle (among others) are all terms that refer specifically to the anatomy of composite flowers. Try googling the term if you’re unfamiliar with it, or search for “composite flowers” here for some relatively useful labelled photographs.
Table 3. Traits for adult plants (post-harvest).
| TRAIT | UNITS | SYSTEM | WHO | HOW |
| whole plant biomass | g | wild and domestic sunflower | UGA/BTM | Harvest all above-ground biomass and place into labelled paper bag(s). Usually the plant will need to be chopped up to fit. Dry at ~60C for at least 4 days, or until biomass reaches a constant weight, then weigh. |
| stem biomass | g | wild and domestic sunflower | UGA/BTM | As above, but separate main stem from all other above-ground biomass, and dry and weigh separately. |
| non-stem biomass | g | wild and domestic sunflower | UGA/BTM | As above, but separate main stem from all other above-ground biomass, and dry and weigh separately. |
| stem density | g/cm2 | wild and domestic sunflower | UGA/BTM | Take a standard length section (10–30 cm) from the basal or apical portion of the stem (either right at stem end, or ~5 cm in from stem ends), removing any branches or leaves. Make sure you have labeled the stem in some way. Place the stem section into a full beaker of water and hold it with a small needle or pointer so that it is completely submerged. Collect the displaced water and record its mass. The stem section should then be placed into a paper bag and dried in a forced air oven at 60C for at least 3-4 days (BTM: usually longer). Once the stem section has reached a constant weight record the dry weight in grams. These dry sections can also be chemically analyzed later. |
| seed set | integer | wild and domestic sunflower | UGA/BTM/NCK | Harvest all flower heads with mature seeds, and either allow to air-dry (min. 1 week) or oven dry (min. 3 days). When dry, remove seeds from heads and count. For domestic plants you can use the lab’s seed sorter and counter for greatly increased efficiency. Unfortunately, these only work for larger seeds. In some cases, you may have two counts: (1) for full, “good” seeds, and (2) for flat, unfertilized, “bad” seeds. |
| seed weight | g | wild and domestic sunflower | UGA/BTM/NCK | Seeds are processed as above and then weighed. Usually weighed in batches of 50 or 100 seeds, and averaged to get per seed weight. |
Contributors (Who):
BTM: Brook Moyers
HCR: Heather Rowe
KGT: Kathryn Turner
NCK: Nolan Kane
UGA: the Knapp and Burke labs at the University of Georgia (local experts: Dan Ebert and I)