HOW DO YOU CALCULATE OSMOLARITY?
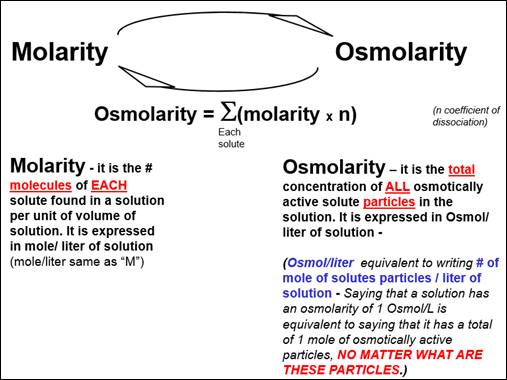
If a
solution contains ONLY ONE type of solute (for example: ONLY
glucose and nothing else or ONLY sodium chloride and nothing else), its osmolarity can be calculated from the following equation:
Osmolarity = molarity x n
n: number of particles that
dissociated from the solute molecule.
For example,
- potassium chloride molecule (KCl) dissociates into
two particles ( K+, Cl--),
- calcium chloride molecule (CaCl2) dissociates into three particles (Ca+,
Cl-, Cl-).
- sucrose (C12H22O11) does not dissociates into particles.
The osmolarity
of solution containing a 1M solution of NaCl is: 1x2 = 2 osmol/L;
The osmolarity
of solution containing a 1M solution of CaCl2 is 1x3 = 3 osmol/L.
The osmolarity
of solution containing a 1M solution of sucrose is 1x1 = 1 osmol/L.
(This equation
can be also used to calculate the osmolarity
of solutions whose solutes that do not dissociate such as sucrose, glucose, urea, glycerol,
.... Their osmolarity equals their molarity
because n=1).
If a
solution contains MANY different type of solutes (for example: BOTH
glucose and sodium chloride are in the solution), its osmolarity can be calculated from the following equation:
Osmolarity = SUM OF ALL (molarity x
n) OF EACH SOLUTE
n: number of particles that dissociated from the solute molecule.
The osmolarity
of solution containing 1M solution of NaCl AND 1M solution of CaCl2 AND 1M
solution of sucrose is 6 osmol/L