The Endomembrane System
 |
Overview: The endomembrane system consists
of the Endoplasmic Reticulum, the Golgi apparatus, Lysosomes, Endosomes
and Secretory Vesicles. These compartments are involved in the processing
of proteins for export from the cell, proteins destined for lysosomes,
and proteins entering the cell from the cell surface. Once proteins enter
the endoplasmic reticulum they never return to the cytosol compartment;
they are carried by vesicle transport to the other compartments of the
system. This flow of vesicles is highly regulated.
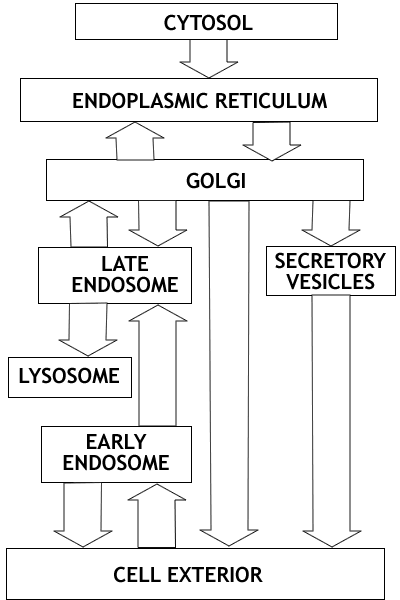
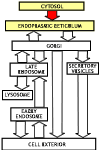
Overview
of the endomembrane system outlining the three major parts of the system.
There are three major subdivisions of the endomambrane system
- the secretory pathway
- the lysosomal pathway and the
- endocytotic pathway
Proteins entering the secretory or lysosomal are synthesized
on ribosomes in the cytosol and are then transferred to the endoplasmic
teticulum |
Protein targeting to the Endoplasmic Reticulum
1. Proteins entering the ER
 |
Overview: Synthesis of all proteins begins
in the cytosol compartment. For proteins entering the secretory or Lysosomal
pathways, the first step is targeting to the endoplasmic reticulum. This
targeting relies on a targeting signal encoded in the N terminal portion
of the protein. The targeting signal is recognized by a specific receptor
that results in the protein entering the endoplasmic reticulum. |
1. Targeting of Proteins to the Endoplasmic Reticulum.
Synopsis. Synthesis of proteins entering the endoplasmic reticulum
is initiated on free ribosomes. A targeting sequence of hydrophobic amino acids
near the amino terminal end of the growing polypeptide results in the binding
of the ribosome to ER membrane and in insertion of the polypeptide into the
endoplasmic reticuluum.
Proteins secretory or lysosomal pathways enter the ER and don't come out again.
The proteins entering either of these pathways may be of either of two types:
- Proteins that are completely translocated into the endoplasmic reticuluum.
These proteins are soluble (not membrane proteins) and are destined for secretion,
or for transfer to lysosomes. In all of these cases the proteins are
never part of membranes.
- Proteins that are inserted into membranes, and hence are only partially
translocated into the endoplasmic reticuluum. These proteins may be destined
for ER, membranes of another organelle (Golgi, lysosomes or endosomes), or
the plasma membrane. In all of these cases the proteins stay within the
membrane once they are inserted into the ER membrane (e.g. cellulose synthase).
Translation of all proteins begins on free
ribosomes. Those ribosomes that produce proteins for export through the
endoplasmic reticulum become attached to the endoplasmic reticulum as ribosomes
of the rough ER. The signal for ER entry is 8 or more hydrophobic amino acid
residues (Table 14-3) which rivets the polypeptide to the ER membrane and is
also involved in translocation.
Whether or not a ribosome becomes attached
to the endoplasmic reticulum depends on the nature of the message being translated,
the protein being made, and is not an intrinsic property of the ribosome itself.
The ribosome and its attached nascent peptide become targeted to the endoplasmic
reticulum.
 Figure 14-12
Figure 14-12
Targeting to the endoplasmic reticulum
takes place through the interaction of the signal peptide sequence
( a sequence of at least eight hydrophobic amino acids at the amino terminal
end of the polypeptide. The emerging signal sequence combines with a 'signal
recognition particle' (SRP). This greatly reduces the rate of translocation
and allows the ribosome to attach to the endoplasm reticulum by means of a special
SRP receptor in the ER membrane.
The ribosome becomes attached to a ribosome
receptor that also functions as the translocation channel
for the newly synthesized polypeptide. As the ribosome becomes attached, the
SRP is removed and translation resumes.
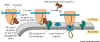 Figure 14-13. shows two components.
Figure 14-13. shows two components.
1. There is a Signal Recognition Particle
(SRP) in the cytosol. This binds to the ER Signal sequence when it is exposed
on the ribosome and slows protein synthesis long enough to allow the SRP to
find the second part, the SRP Receptor.
2. The Signal Recognition Particle Receptor (SRPR) which is embedded in the
ER membrane. We now have the new polypeptide synthesizing system in place and
protein synthesis speeds up. It seems that the Signal Sequence opens the translocation
channel.
Experimental test that ER targeting signal is both necessary and sufficient
to bring about targeting.
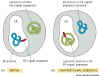 |
Figure 14-6 experimental test of the role of signal sequences.
IMPORTANT |
How do proteins get into the ER?
SOLUBLE PROTEINS
The peptide moves through the translocation
channel into the lumen of the ER. The signal peptide sequence remains attached
to the membrane. It is later cleaved off by a signal peptidase.
Leaving the protein free in the lumen of the ER.
MEMBRANE PROTEINS
Key point is that the orientation of a protein
in the membrane is established when it is first inserted into the membrane.
This orientation of the protein persists all of the way to its final destination.
That is, the cytosolic side of membrane remains on the cytosolic side throughout
all processes.
As membrane proteins are being translated,
they are translocated or transferred into the ER until a hydrophobic membrane
crossing domain is encountered. This serves as a 'stop transfer' signal and
leaves the protein inserted in the ER membrane.
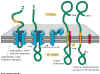
Figure 14-15.
Animation
of this process
|
Import of a
membrane protein. This figure illustrates the case of a protein being incorporated
in the membrane of the endoplasmic reticulum, but import into organellar
membranes works much the same way. The blue sheath-like component shown
in the figure is the transport complex that moves the protein through the
membrane. This example is a single pass membrane protein that contains a
single membrane crossing domain. |
The hydrophobic trans-membrane domain holds
the protein in the membrane because of the very strong hydrophobic interaction
between this part of the protein and the hydrophobic membrane core.
Try this excellent link to web site dealing
with insertion of
proteins in membrane.
Proteins with multiple membrane crossing domains
are inserted in the the membrane through the action of multiple pairs of start
transfer and stop transfer signals:
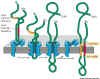
Click to enlarge
Animation
of this process
|
Fig. 14-16 insertion of a
double pass membrane protein into the membrane. The signal sequence is not
at the N terminus and is not removed. Transfer continues until a stop signal
is reached. There may be more than one pair of start and stop transfer
signals. Transfer is reinitiated with each start transfer signal. This means
that at each transfer stop signal (membrane crossing domain) the ribosome
becomes detached from the ER membrane. If later a start transfer sequence
is encounters, it binds to a new SRP and forms a new association between
the ribosome and the ER membrane that leads to the insertion of the start
transfer sequence and the following amino acids up to and including either
the C terminal end or a stop transfer sequence, which ever is encountered
first. |
There are two major categories of hydrophobic
signals used in insertion of membrane proteins. All of these are membrane crossing
domains:
- Start transfer sequences. These
are of two types:
- N-terminal signal peptide sequence
- a cluster of about 8 hydrophobic amino acids at the N-terminal end of
a protein. This sequence remains in the membrane and is cleaved off of
the protein after transfer through the membrane.
- Internal start transfer sequence.
Similar to a signal sequence, but located internally (not at the N terminal
end of the protein). It also binds to the SRP and initiates transfer.
Unlike the N-terminal signal sequence, it is not cleaved after transfer
of the protein.
- Stop transfer signal. This is also
a sequence of about 8 hydrophobic amino acid residues. It follows either a
N-terminal signal sequence or a start transfer sequence. The stop transfer
signal is a membrane crossing domain. It remains in the membrane. The peptide
is not cleaved.
This process of membrane insertion has a very
important result: It establishes orientation of membrane proteins. Recall
the earlier discussion of 'sidedness of membranes'. This is one of the
chief ways that 'sidedness' happens.
Notice that the C-terminal end of the protein
is on the cytosolic side of the membrane and the N-terminal end is not in the
cytosol, but on the inside of the ER, or organelle.
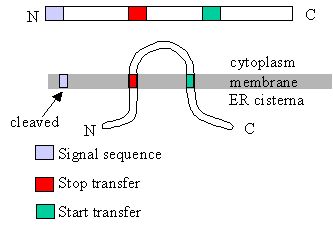 |
This diagram shows the relation
between translocation control sequences (signal sequences, start transfer
sequences, stop transfer sequences) and the arrangement of the protein in
the membrane. How would the translocation control sequences have to be arranged
to get the N terminal end of the protein on the cytoplasmic side?
- to get the C terminal end of the protein on the cytoplasmic side? |
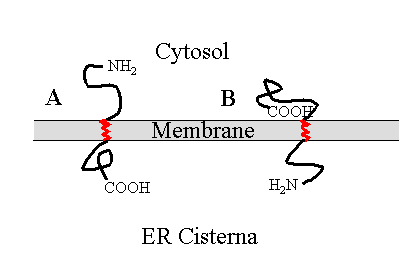
Now look at what happens if the protein is
incorporated into a vesicle and later fused with the plasma membrane: The
cytosolic side remains in the cytosol. This is a key idea.
 |
Insertion of membrane proteins and sidedness of membranes.
In A, a protein is inserted into the ER membrane. The cytosolic side
of the membrane is labled 'C'. If a vesicle is budded off of the ER containing
the protein, the cytosolic portion is still in the cytosol (Figure B).
If the vesicle fuses with the plasma membrane (Figure C) the same
relationships are maintained, resulting in the incorporation of the protein
into the plasma membrane (Figure D) with the same orientation (C
vs I) that it had when it first entered the membrane. |
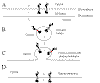
 |
Can you do this one?
Make a map of each of the proteins shown at A and B in the figure at
the left. Indicate N terminal end, the relative location of any signal
sequences. start transfer sequences, stop transfer sequences, and the
C terminal end. The membrane crossing domains are shown in red.
Explain the sequence of events leading to insertion of each of these
proteins in the membrane (This is a very important test of understanding)
See Fig. 14-15 and 14-16
for hints. |
|
Aside: On Collagen
Pages 601-602
Just a word about the protein collagen,
which may form more that 50% by weight of certain tissues in your body.
This is an extracellular fibrillar polymer, which has some similar functions
to cellulose in plants, but which is built of protein (not polysaccharide).
The textbook tells you some neat things
about this molecule, which is neat especially if you are , for example,
double-jointed.
The textbook does not tell you of the
relevance to collagen to our present topic.
Collagen differs from other most other
proteins, but is similar to a few others like keratin and elastin, because
it contains two modified amino acids (hydroxyproline and hydroxylysine).
Both of these amino acids are coded normally on the rough ER, as proline
and lysine BUT as they are transferred to the ER, some of these amino
acids are hydroxylated by an enzyme which is part of the translocation
machinery in the ER membrane.
So, it is important to understand that
translocation may also include modification. We believe that the signal
for this is in the protein sequence, where pro-collagen contains many
repeats Pro.Pro.Gly. and usually the first Pro is the one that is modified.
The translated sequence is 30% composed of pro pro gly
with about 6% lys. As collagen is made and imported into
the ER about 40% of the pro is hyrozylated to form 4 hydroxyproline. The
enzyme involved is proline hydrozylase. This enzyme is located in
the ER membrane, associated with RER. The resulting sequence in
collagen is hyp pro gly some hydroxy lysine is formed also.
|