See also this post by Kathryn (Dan E.).
Goal. Convert area to pixels. Measure each pixel for precise area measurement of odd shapes (leaves, seeds etc.) without bias. I’ve included a practice image of an Arabidopsis stem.
Materials:
Paper ruler: http://www.vendian.org/mncharity/dir3/paper_rulers/
ImageJ: http://rsbweb.nih.gov/ij/ or sudo apt-get install imagej (on Debian-like systems)
1. Print ruler without page scaling.
2. Scan your plant tissue (leaf, seed, stem*) on a paper ruler. At least 300 dpi. Over 600 dpi is overkill. Try and use a single paper ruler rather than a page of ruler like I used in the example provided.
3. Start ImageJ and drag in your image(s)
4. I like to crop my images to only the area containing tissue (it helps on my lower powered laptop).
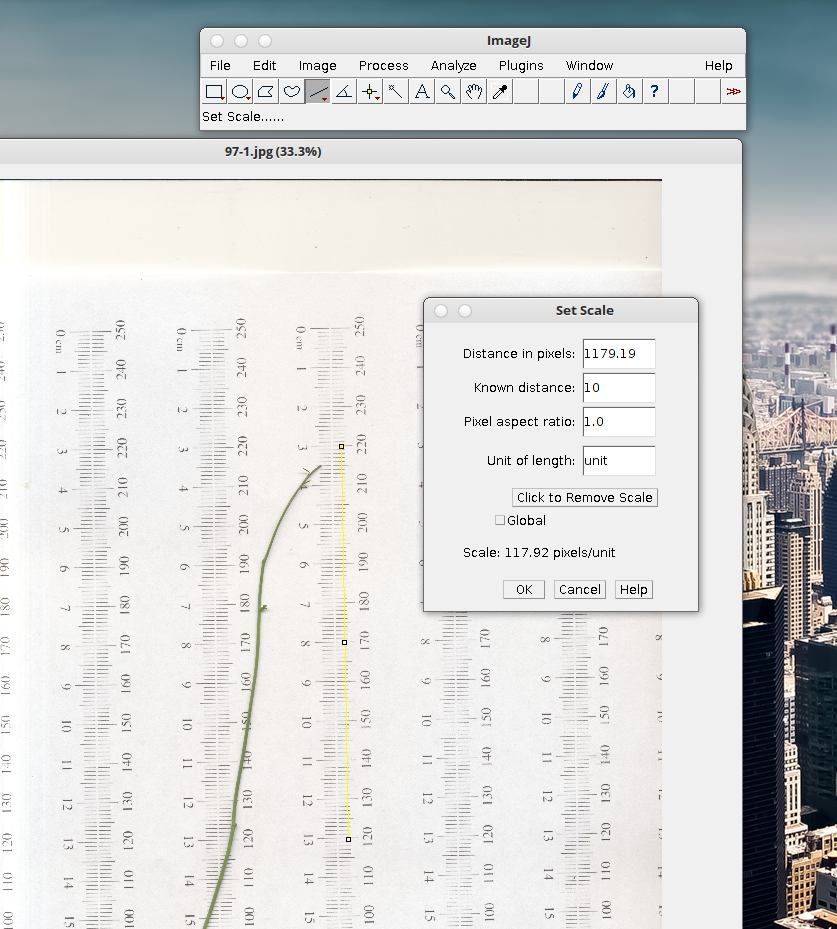
5. Select the line tool and draw a line from one point on the ruler to another. Try to make it as perpendicular to the mm markers as possible. If you have a yellow line with 2 or 3 open squares you’re set.
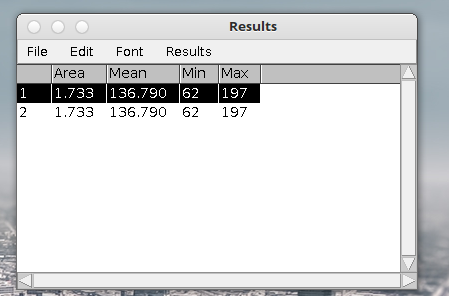
6. Select Analyze –> Set scale. Enter your known line length. In this case it’s 10 cm. At 300 dpi the pixel length is ~ 120 pixels cm. Click global if you want to retain the calibration in memory for multiple images.
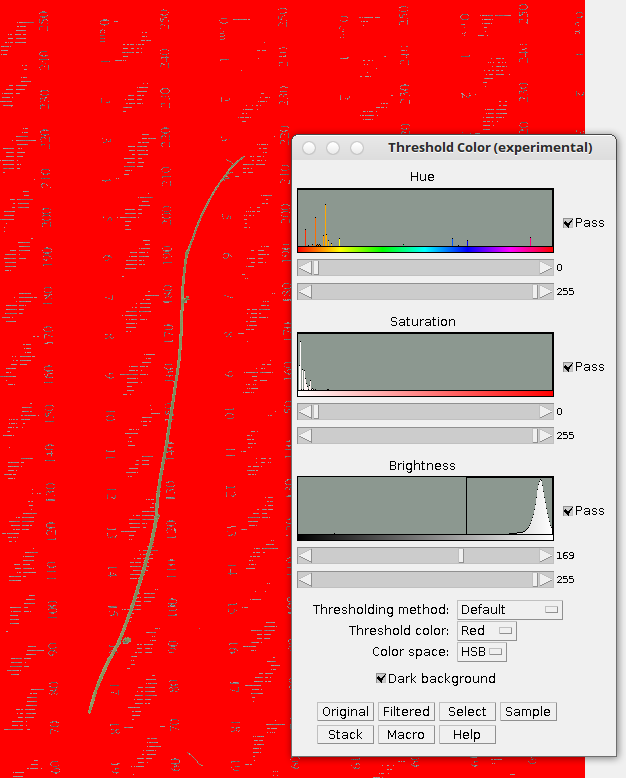
7. Choose Image –> Adjust –> Color Threshold
When you alter the hue, saturation and brightness you’ll cause the background to exclude the plant tissue. In this case the green stem becomes distinct from the red background. The background color choice is entirely up to you.
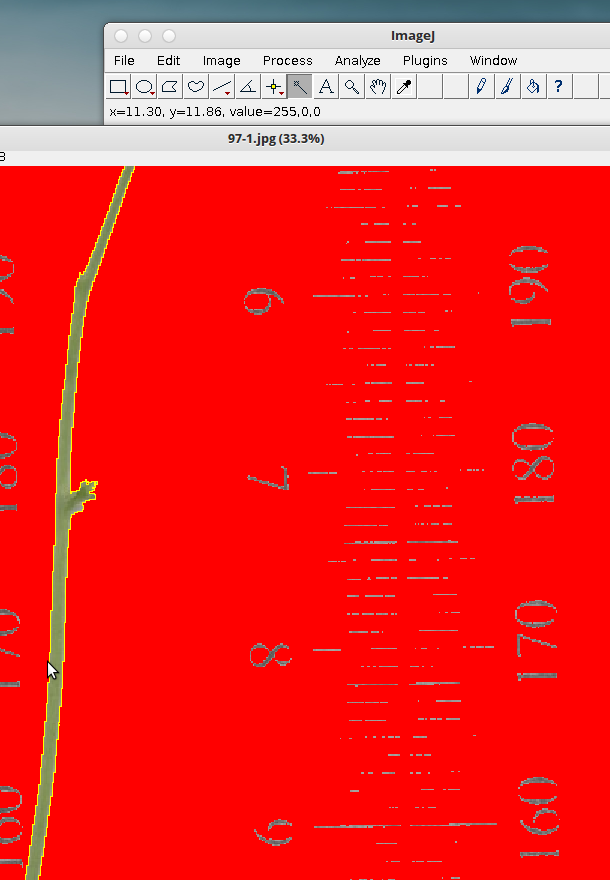
8. Select the wand (tracing)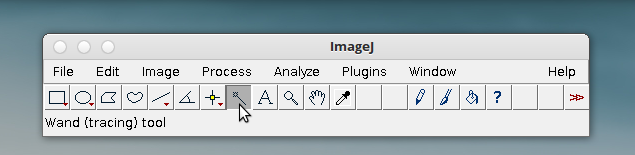 tool.
tool.
Now double click on the edge of your tissue. The edge is the junction between the red background and the green tissue. It’s a little bit fiddly at times. Don’t be frustrated if it doesn’t work right away.
It helps to zoom in (press the + key to zoom in)