Chapters
Lodish 4th edition: Chapter 21 pages 951-959
Moyes and Schulte: Chapter 7 pages 250-253; 261-264.
Introduction to Sensory receptors
Organisms use their sensory systems to gain information about their
environment. External cues are converted or transduced into information that
the organism uses to survive.
From Shephard (1994) Neurobiology 3rd edition.
Specificity
Each sensory system is specific to the type of information or external cue that is able to detect. So for instance the visual system is designed to detect photons and not to detect chemical odorants. This specificity arises from the sensory endings that are used by each system. Again in the visual system the photoreceptors are designed to trap and respond to photons of light. The olfactory system contains receptors designed to respond to single or limited numbers of chemical odorants. The taste system also detects a range of chemicals though more limited than the olfactory system. There are also mechnosensory systems that detect and transduce mechanical force into a neurnal signal.
While most animals share these systems there are some unique specializations. Some snakes for instance have photoreceptors capable of responding to the infra-red spectrum of light (presumably for hunting warm blood prey at night). Primates and humans are capable of distinguishing colours. Insects are capable of detecting ultra-violet light.
Location
Many sensory systems are capable of distinguishing the location of the
stimulus such as touch, light or odour.
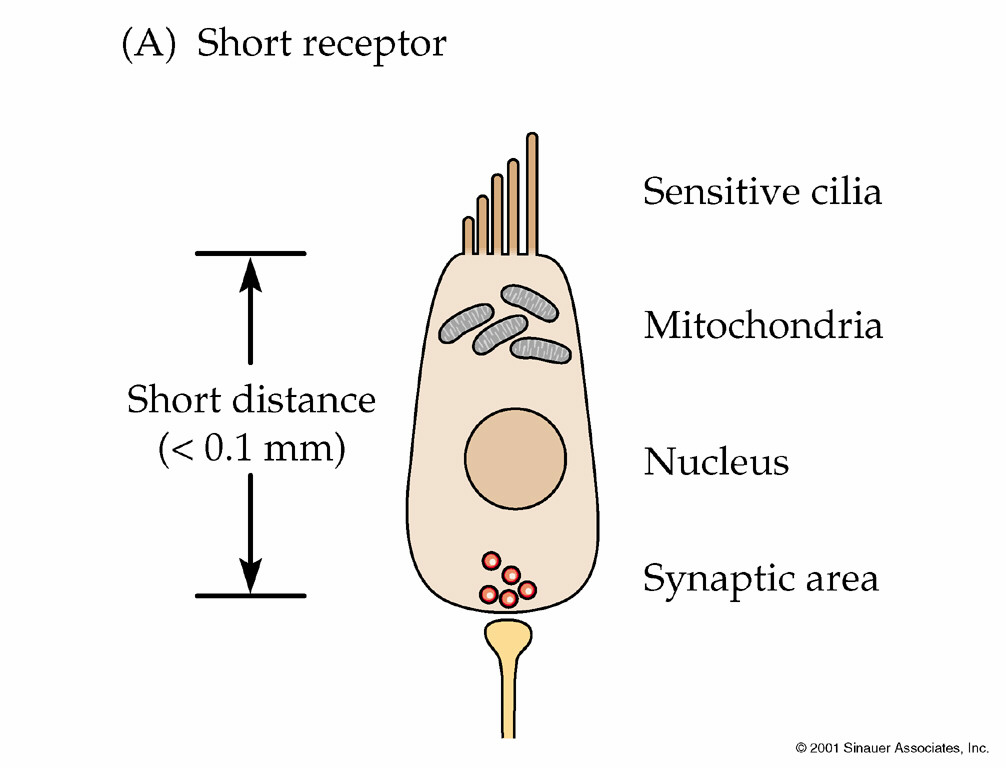
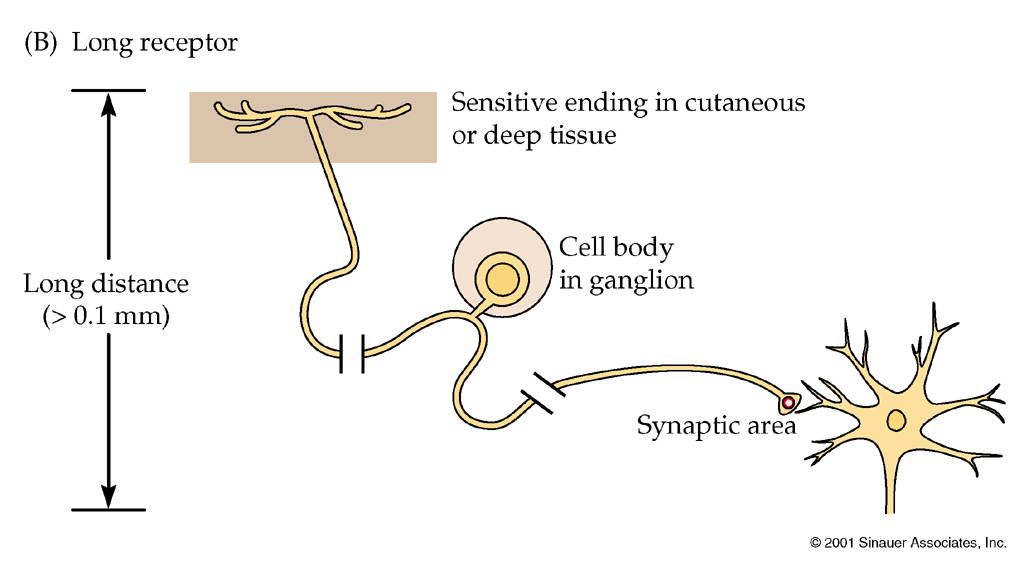
From Nicolls, From Neuron to Brain
Duration
Each sensory system can determine the length of the stimulus by responding to the stimulus for the duration or at the beginning and the end of the stimulus. For instance cold sensitive somatosensory neurons will have an increased firing rate for the duration of the cold stimulus. Other types of mechanosensory neurons will respond to a touch at the beginning and the end of the stimulus therefore encoding information as to the length of the stimulus.
Intensity
The intensity of the stimulus can be encoded by an increase in action
potential frequency in some systems or by an increased neurotransmitter
release in other systems.
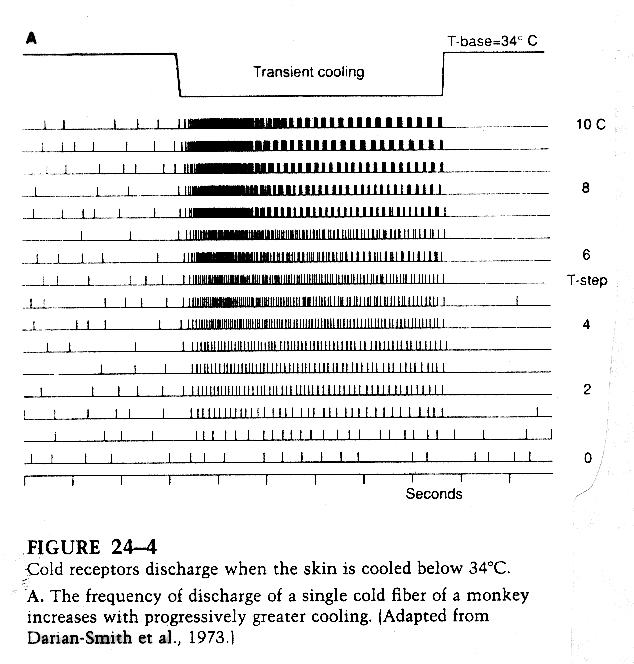
From Kandel, Principles of Neural Science
Intensity Encoding
In the somatosensory system the intial depolarization is graded depending on
the intensity of the stimulus. For instance in the mechanosensory neurons a
touch will open mechano-gated Na+ channels which will create a depolarization.
The greater the degree of touch (or deflection of the receptor) the greater
the depolarization.
This is accomplished by opening a greater number of receptors or opening
receptors for longer. As the depolarization increases in neurons that fire
action potentials, the frequency of action potentials increases.
How does this come about?
As the depolarization increases the ability to overcome the relative refractory period increases. The greater the depolarzation the early in the relative refractory period another action potential can be fired. The depolarization has to overcome both the hyperpolarization generated by the delayed rectifier and the reduced number of voltage-gated Na+ channels available to be opened (many will still be inactivated from the previous action potential).
Chemical Senses
Both the olfactory and taste senses are chemical senses. In other words
these systems have receptors that can detect chemicals in the environment. In
mammals these receptors are found either on the tongue for taste or in the
nasal cavity for olfaction.
We will discuss taste receptors as examples of chemical senses as this sense
is essential for survival of many animal species and covers the broadest range
of examples of sensory transduction.
Taste chemical transduction is the ability to detect water soluble non-volitile chemical compounds at fairly high concentrations. This is different from olfaction which is very sensitive (i.e. can respond to small concentrations) and detects volitile air-borne chemical. Both chemical senses play an important role in the survival of the animal. Taste for detection of salts, sugar, toxins etc. Smell for food detection, mate selection, nuturing of offspring.
Taste transduction.
Taste cells or taste receptors are found in many different areas, in
mammals in the tongue and mouth, in insects on the pads of feet and in fish
along the body.
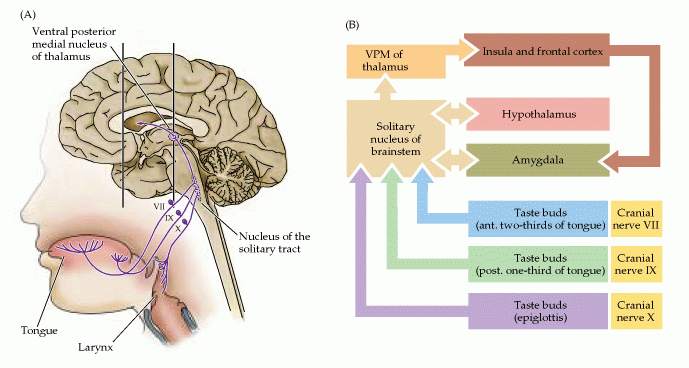
Figure 15.10. Organization of the human taste system.
(A) Drawing shows the relationship between receptors in the oral cavity and upper alimentary canal, and the central nervous system. (B) Diagram of the basic pathways for processing taste information.
We will concentrate on taste cells in mammals. The tongue contains taste buds
that are spread along the edges and back of the tongue. Another set are found
at the back of the throat (epiglottis).
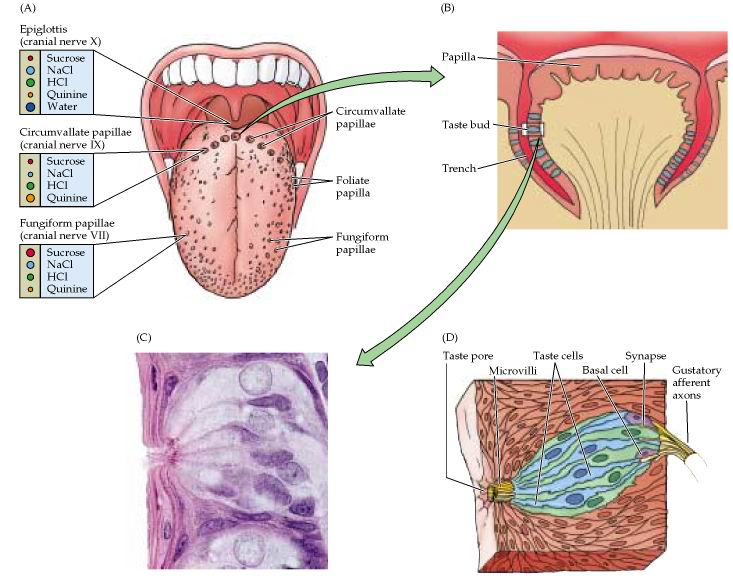
Figure 15.11. Purves, Neuroscience 2nd edition.
Taste buds and the peripheral innervation of the tongue. (A) Distribution of taste papillae on the dorsal surface of the tongue. Different responses to sweet, salty, sour, and bitter tastants recorded in the three cranial nerves that innervate the tongue and epiglottis are indicated at left. The size of the circles representing sucrose, NaCl, HCl, quinine, and water corresponds to the relative response of the papillae to these stimuli. (B) Diagram of a circumvallate papilla showing location of individual taste buds. (C) Light micrograph of a taste bud. (D) Diagram of a taste bud, showing various types of taste cells and the associated gustatory nerves. The apical surface of the receptor cells have microvilli that are oriented toward the taste pore.
Each taste bud is made up of 50 to 150 taste cells. These cells are specialized epithelial cells and thus have apical and basal sides and are joined by tight junctions. Each cell has an average life span of 10-14 days. There are basal stem cells in each taste bud that divide to regenerate the taste cells.
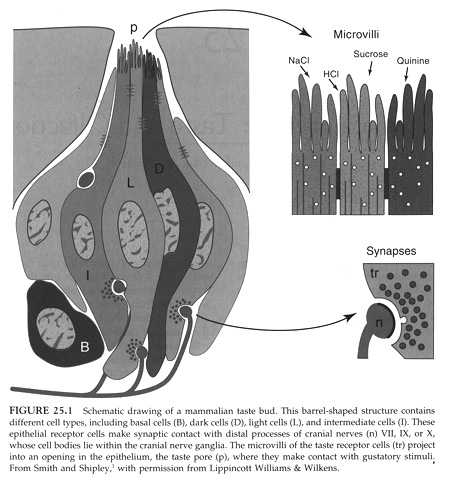
From Sperelakis, Cell Physiology 2nd edition
Each taste cell has microvilli on its apical surface that project up into
the mucus of the tongue. The taste receptor proteins are found in the
microvilli and thus the chemicals are soluble and diffuse to the bind to their
receptors. Each taste bud can be made up of multiple taste cells with
different chemical sensitivities. For instance different cells in the same bud
can detect NaCl, sucrose, H+ and quinine (bitter).
Each taste cell forms a chemical synapse with a sensory neuron that projects
to the brain from the tongue. (The neurons travel in the facial (VII) and
glossopharyngeal (IX) cranial nerves - this is a cocktail party fact only).
The sensory neurons can receive information from different taste cells and
thus respond to a combination of chemicals.
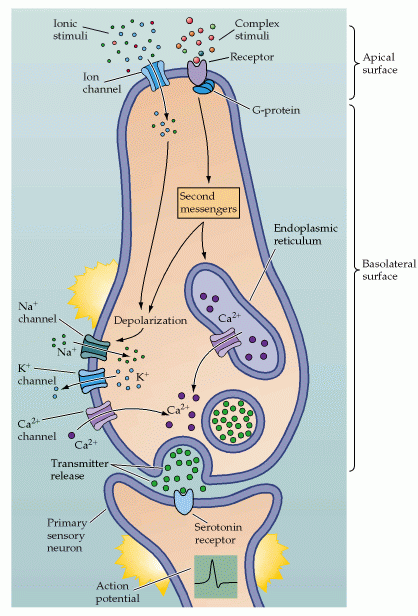
Figure 15.12. Purves, Neuroscience 2nd edition.
Transduction mechanisms in a generic taste cell. The apical and basolateral surfaces of the cell are separated by tight junctions. The apical surface contains both channels and G-protein-coupled receptors that are activated by chemical stimuli. The basolateral surface contains voltage-gated Na+, K+, and Ca2+ channels, as well as all the machinery for synaptic transmission mediated by serotonin. Also shown are the relevant second messenger systems and intracellular compartments that store Ca2+. The increase in intracellular Ca2+ either by the activation of voltage-gated Ca2+ channels or via the release from intracellular stores causes synaptic vesicles to fuse and release their transmitter onto receptors on primary sensory neurons.
Each cell contains the standard complement of neuronal proteins including Na+/K+ ATPase at the basal level, voltage-gated Na+ and Ca+2 channels, leak K+ channel. The response to the chemical is mediated by the expression of receptors for that chemical in the microvilli. The response is a depolarization of the cell sometimes enough to generate an action potential. The signaling of the cell to the sensory neuron depends on a sufficient depolarization to open the voltage-gated Ca+2 channels necessary for vesicle fusion and neurotransmitter release.
There are five major tastes in animals including salt, sweet, sour, bitter
and amino acids.
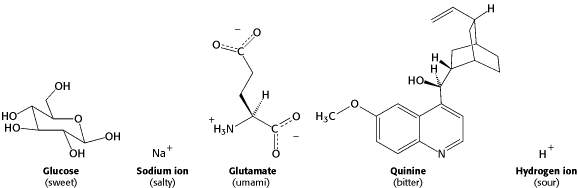
Figure 32.11. Stryer Biochemistry 5th edition. Examples of Tastant Molecules. Tastants fall into five groups: sweet, salty, umami, bitter, and sour.
Each type of taste receptor cell will be outlined below:
Salt taste cells.
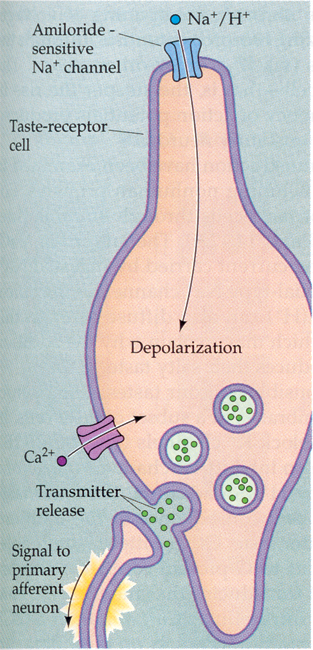
From Purves, Neuroscience 2nd edition
The Na+ enters into the cell through the passive amiloride-sensitive Na+
channel. This channel is a member of a large family of conserved proteins that
are all blocked by amiloride. These proteins are found in frog skin and
kidney.
Amiloride will block Na+ salt taste reception. Entry of Na+ into the cell of
course causes the cell to depolarize. Need a large concentration of Na+ to
trigger a sufficient depolarization to signal to the post-synaptic sensory
neuron.
Sour taste cells.
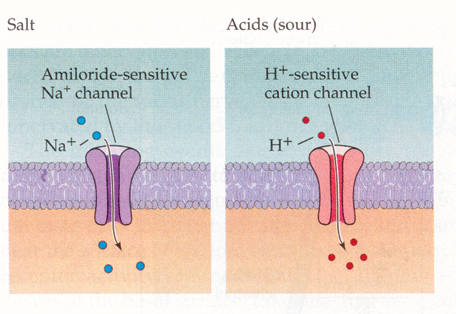
From Purves, Neuroscience 2nd and 3rd editions
Taste response produced by acids, excess protons. These positive ions enter the cell through a H+, cation specific ion channel and in turn depolarize the cell to threshold for an action potential.
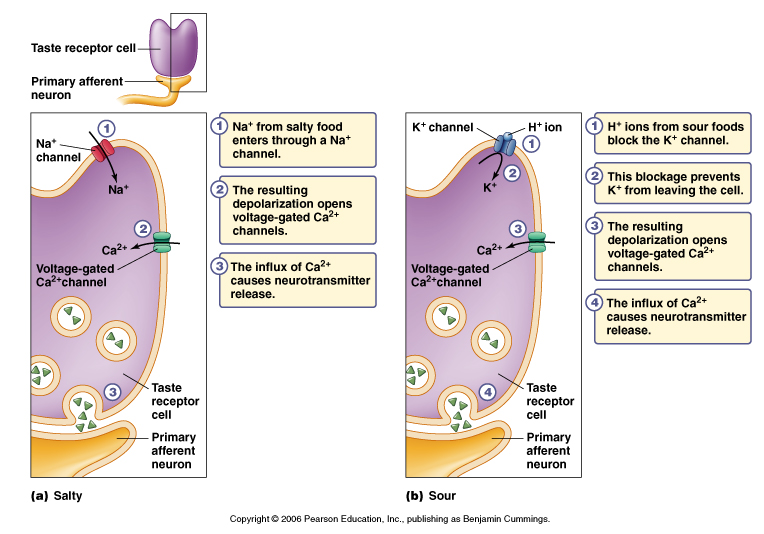
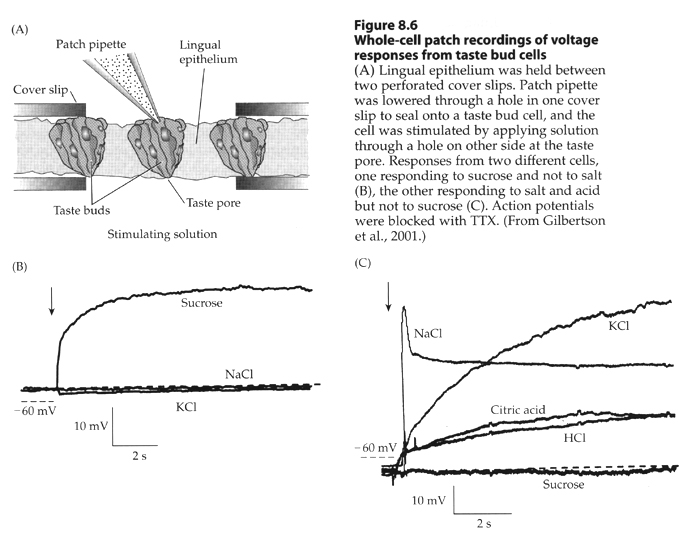
Figure 7.11ab Signal transduction in taste receptor cells.
From Moyes and Schulte.
Sweet taste cells
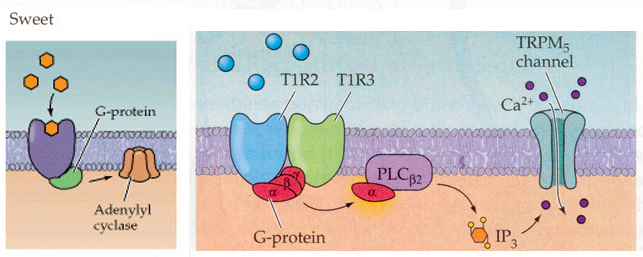
From Purves, Neuroscience 2nd and 3rd editions.
There are specific membrane receptors for different sweeteners and sugars.
These receptors are not ligand gated ion channels but rather are metabotropic
receptors. These receptors belong to the family of seven transmembrane domain
proteins that are linked to signaling cascades through G proteins.
In mammals a combination of the T1R2/T1R3 receptors have a response to sugars
and sweeteners. These receptors stimulate a G protein (Gq) which in this case
activates phosopholipase C (PLC). PLC as you know breaks down PIP2 (phosphatidylinositol
4,5-bisphosphate) into IP3 (inositol triphosphosphate) and DAG (see Figure
13-28 in Lodish 5th edition for review). In particular IP3 will bind to and
activate a ion channel (TRP channel called TRPM5) which allows Ca+2 to influx
into the cell. In other words this pathway leads to a depolarization and
threshold is reached to trigger an action potential.
In other animals sugars also appear to bind to receptors that stimulate G proteins (Gs) that activate adenylate cyclase. This results in an increase in cAMP in the cell that activates a protein kinase (PKA) which in turn phosphorylates a K+ channel to close the channel. Once the K+ channel is close the cell will depolarize.
Both these signaling cascades are used in multiple biological systems. In the nervous system neurotransmitter binding to specific metabotropic receptors can trigger these cascades. Photoreceptor and olfactory neurons also use parts of these cascades for their sensory transduction.
Bitter taste cells.
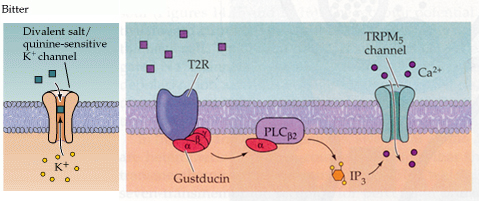
From Purves, Neuroscience 2nd and 3rd editions
Different cells have different mechanisms of bitter taste transduction.
1. In mammals the bitter receptor is a metabotropic receptor called T2R. There
are about 30 different subtypes in mammals. These signal through a G protein
called gustducin to PLC and thus generate IP3. Again like sweet receptors the
IP3 activates a TRPM5 channel to open and allow Ca+2 to influx into the cell.
2. Some bitter chemicals such as quinine bind to and block specific K+
channels and thus result in depolarization of the cell.
Of note is that the gustducin G protein is very conserved with a G protein found in the photoreceptors (transducin) that does exactly the same thing i.e. activate a phosphodiesterase (PDE). Knock out mice that lack gustducin fail to detect some (but not all) bitter compounds
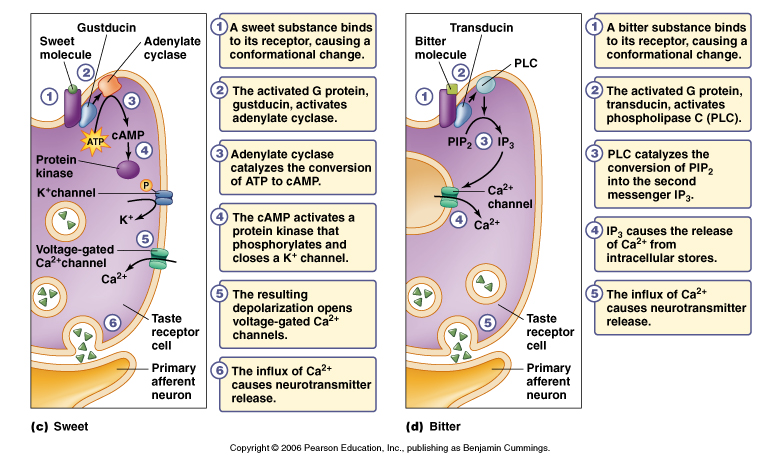
Figure 7.11cb Signal transduction in taste receptor cells
From Moyes and Schulte
Amino acid taste cells.
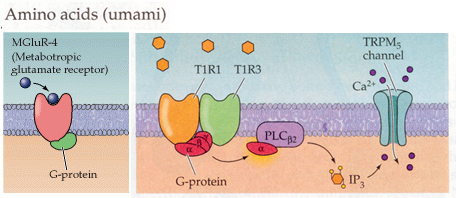
From Purves, Neuroscience 2nd and 3rd edition
In some animals catfish for instance there are a high number of amino acid
taste cells. There appears to be multiple ways that animals respond to amino
aicds. 1. In fish and other amphibians, amino acids such as L-arginine and L-proline
bind to specific receptors which are ligand gated ion channels (in precisely
the same manner as the acetylcholine and glutamate ligand gated ion channels
work that we discussed in section 2).
2. In mammals there are taste cells that respond to L-glutamate. In these
cells L-glutamate activates a metabotropic receptor glutamate receptor linked
to a G protein. Glutamate binds to many different metabotropic receptors and
in taste cells it is the mGluR4 that is responsible for the taste
transduction.
3. In mammals there are also two metabotropic receptors T1R1/T1R3 that combine
to respond to the standard 20 amino acids. This combination signals through G
protein activation of PLC and the generation of IP3 and the activation of the
TRPM5 channel.
From Fain, Sensory Transduction
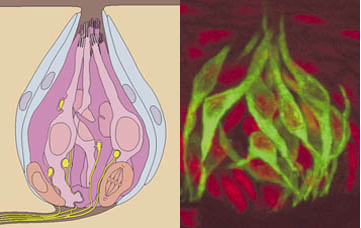
Image showing the localization of gustducin (green) in taste bud cells