Chapters
Lodish 4th edition: Chapter 18
Lodish 5th edition: Chapter 5
Moyes and Schulte: Chapter 6 pages 239-243
Moyes and Schulte: Chapter 9 pages 380-381
Smooth Muscle cells

Figure 22-40, Molecular Biology of the Cell, 4th edition. The four classes of muscle cells of a mammal.
(A) Schematic drawings (to scale).
(B-E) Scanning electron micrographs, showing (B) skeletal muscle from the neck of a hamster, (C) heart muscle from a rat, (D) smooth muscle from the urinary bladder of a guinea pig, and (E) myoepithelial cells in a secretory alveolus from a lactating rat mammary gland. The arrows in (C) point to intercalated discs—end-to-end junctions between the heart muscle cells; skeletal muscle cells in long muscles are joined end to end in a similar way. Note that the smooth muscle is shown at a lower magnification than the others.
Smooth muscles surround internal organs such as the large and small intestines, the uterus, and large blood vessels. The contraction and relaxation of smooth muscles controls the diameter of blood vessels and propels food along the gastrointestinal tract.
Smooth muscle cells are very heterogeneous i.e. multiple different types. They are characterized by an assembly of short, narrow cells with differing properties. Compared with skeletal muscles, smooth muscle cells contract and relax slowly, and they can create and maintain tension for long periods of time.

Figure 22-22. Molecular biology of the cell. Diagram of a small artery in cross section.
A smooth muscle is composed of elongated spindle-shaped cells, each with a single nucleus. Although smooth muscle cells are packed with thick and thin filaments, these filaments are not organized into well-ordered sarcomeres and thus smooth muscle is not striated. Instead the filaments in smooth muscle are gathered into loose bundles, which are attached to dense bodies in the cytosol. Dense bodies apparently serve the same function as Z disks in skeletal muscle. The other end of the thin filaments in many smooth muscle cells is connected to attachment plaques, which are similar to dense bodies but are located at the plasma membrane of a muscle cell. Like a Z disk, an attachment plaque is rich in the actin-binding protein alpha-actinin; it also contains a second protein, vinculin, which binds to an integral membrane protein in the plaque and to alpha-actinin, thereby attaching actin filaments to membrane adhesion sites.
Figure 18-26, Lodish 4th edition. General structure of skeletal and smooth muscle.
(b) Smooth muscle is composed of loosely organized spindle-shaped cells that contain a single nucleus. Loose bundles of actin and myosin filaments pack the cytoplasm of smooth muscle cells. These bundles are connected to dense bodies in the cytosol and to the membrane at attachment plaques.

From Cell Physiology, Sperelakis, 2nd edition.
The sliding filament model is still used in smooth muscle contraction. However in smooth muscle cells contraction is much slower than skeletal muscle cells. The smooth muscle cells will shorten due to contraction and thus generate tension.

From Cell Physiology, Sperelakis, 2nd edition.
In smooth muscle cells, the sarcoplasmic reticulim network is sparse, and the majoirty of the increase in cytosolic Ca2+ needed for muscle contraction enters the cell via the plasma-membrane Ca2+ channel. This is similar to what occurs in invertebrate, small vertebrate, cardiac cells. This means that changes in the cytosolic Ca2+ level occur much more slowly in smooth muscle (seconds to minutes). This has the advantage of allowing the slow, steady response in contractile tension that is required by vertebrate smooth muscle.
Activation of smooth muscle cells
Smooth muscle cells have multiple receptors and activation mechanisms.
Smooth muscle cells can be activated by neurotransmitters, hormones,
neighbouring cells. For instance, electrical coupling through gap junctions
synchronizes the contractions of the smooth muscle cells responsible for the
peristaltic movements of the intestine.
However the overall goal is always the same....change levels of cytosolic Ca+2
to change the degree of contraction.
Within a single organ and sometimes within a small part of an organ, smooth muscles cells can be contracting, relaxing, and releasing signals to carry out functions. For example with in a blood vessel there are spontaneously active pacemaker cells which can be conducted across a few or many cells.
Some smooth muscle cells have fast contractions while other are slower or maintain muscle tone or sustained contractions for long periods of time. As this a low energy costs there must be mechanisms to allow for the maintence of tension across the cell that are specialized from skeletal muscle cells.

From Cell Physiology, Sperelakis, 2nd edition.
Contraction in some smooth muscle cells are controlled by changes in membrane potential and some are purely through chemical/hormone processes. Nerve innervation of smooth muscle cells is from the autonomic nervous system and similar to cardiac muscle cells works over a wide area of general neurotransmitter release. The function of neurotransmitters is usually to modulate contraction rather than initiate contraction (many smooth muscle cells as stated above have the ability to spontaneously activate). Contractions can occur over minutes rather than milliseconds as was seen with skeletal and hundreds of milliseconds as was seen with cardiac cells.
Smooth muscle cell contraction
Smooth muscle contraction is not controlled by the binding of Ca+2 to the troponin complex as it is in cardiac and skeletal muscles. Rather Ca+2 control myosin attachment to the actin through an intermediate step of Ca+2/calmodulin and it is this that controls contraction in smooth muscle cells. Troponin is not found in smooth muscle cells (tropomyosin is).
Caldemon and Ca+2/calmodulin
Figure 18-33, Lodish 4th edition. Ca2+-dependent mechanisms for regulating contraction in smooth muscle.
(b) Regulation of smooth muscle contraction by caldesmon. At low Ca2+ concentrations (10-6 M), caldesmon binds to TM and actin, reducing the binding of myosin to actin and keeping muscle in the relaxed state. At higher Ca2+ concentrations, a Ca2+-calmodulin complex binds to caldesmon, releasing it from actin; thus myosin can interact with actin and the muscle can contract. Phosphorylation by several kinases, including MAP kinase, and dephosphorylation by phosphatases also regulate caldesmon’s actin-binding activity.
The activation of smooth muscle myosin can be regulate by caldesmon which in low Ca+2 levels binds to tropomyosin and actin and blocks myosin binding to actin. As Ca+2 levels increase Ca+2 activated calmodulin to bind to caldesmon which releases it from the tropomyosin/actin complex. Now myosin is free to bind and move along the thin filaments to contract the cell.
Myosin light chain kinase and Ca+2/calmodulin
Another mechanism of smooth muscle contraction requires the regulation of the light chains that are associated with the myosin heavy chain
Figure 18-34, Lodish 4th edition.
(b) In vertebrate smooth muscle, phosphorylation of the myosin regulatory light chains on site X by Ca2+-dependent myosin LC kinase activates contraction. At Ca2+ concentrations ">"10-6 M, the myosin LC kinase is inactive, and a myosin LC phosphatase, which is not dependent on Ca2+ for activity, dephosphorylates the myosin LC, causing muscle relaxation.
THe activation of smooth muscle myosin requires the phosphorylation of the myosin light chain. There are two enzymes that control this process, myosin light chain kinase (MLCK) and myosin light chain phosphotase. One of the two myosin light chain pairs associated with the myosin in smooth muscle inhibits actin stimulation of the myosin ATPase activity at low Ca2+ concentrations. Phosphorylation of the myosin light chain by MLCK removes this inhibition and the smooth muscle contracts. MLCK is activated by Ca2+ through calmodulin. Calcium binds to calmodulin, and the Ca2+-calmodulin complex then binds to myosin LC kinase and activates it. Because this mode of regulation relies on the diffusion of Ca2+ and the action of protein kinases, muscle contraction is much slower in smooth muscle than in skeletal muscle. The greater the amount of intracellular Ca+2 the more MLCK is activated and the greater the degree of contraction
The role of activated MLCK was proved by injecting a kinase inhibitor into smooth muscle cells. The inhibitor did not block the rise in the cytosolic Ca2+ level associated with membrane depolarization (measured by Fura-2), but the injected cells cannot contract.
The effect of the kinase inhibitor was then overcome by injecting a fragment of MLCK that is always active (constitutively active) even in the absence of Ca2+-calmodulin (this treatment also does not affect Ca2+ levels).
Regulation of smooth muscle contraction
Given the broad diversity of smooth muscle cells there are many means to modulate smooth muscle contraction. For this course examples of control of blood vessels and arterioles will be used.
The major means that control smooth muscle contraction is controlled is
through changes in resing membrane potential.
Depolarization causes a greater increase in cytosolic Ca+2 and thus greater
contraction.
Hyperpolarization causes a reduced amount of cytosolic Ca+2 and thus relaxes
the muscle cell.
However it is important to note that release of Ca+2 from internal stores may
also lead to greater contraction through G protein mediated cascades that have
nothing to do with changes in membrane depolarization.
Norepinephrine and epinephrine
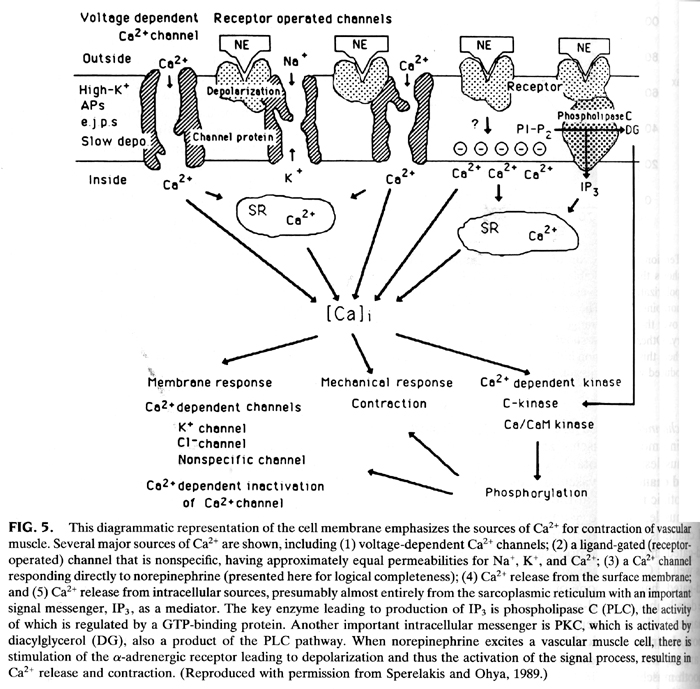
From Cell Physiology, 2nd edition.
Depending on the type of receptor norepinephrine and epinephrine can have
different results on the smooth muscle cell.
Epinephrine bound to beta-adrenergic receptors on smooth muscle cells of the
intestine causes them to relax. Think of a usual biological response to times
of intense stress, i.e. right before a public oral presentation
Epinephrine also binds to the alpha2-adrenergic receptor found on smooth
muscle cells lining the blood vessels in the intestinal tract, skin, and
kidneys. Epinephrine bound to alpha2 receptors causes the arteries to contract
(constrict), reducing circulation to these organs. This response supplies the
maximal amount of energy to the major locomotor muscles in response to bodily
stress.
Acetylcholine and Nitric Oxide
Acetylcholine is released by autonomic nerves in the walls of a blood vessel, and it causes smooth muscle cells in the vessel wall to relax. The acetylcholine acts indirectly by inducing the nearby endothelial cells to make and release NO, which then signals the underlying smooth muscle cells to relax.

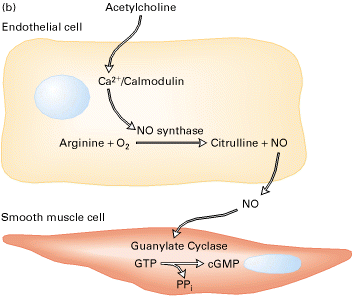
Figure 20-42, Lodish 4th edition. cGMP mediates local signaling by nitric oxide.
(a) Schematic diagram of the structure of soluble guanylate cyclase. Binding of nitric oxide to the heme group stimulates the enzyme’s catalytic activity, leading to formation of cGMP from GTP.
(b) Regulation of contractility of arterial smooth muscle by NO and cGMP. Nitric oxide synthesized in endothelial cells diffuses locally through tissue and activates guanylate cyclase in nearby smooth muscle cells. The resulting rise in cGMP leads to the relaxation of the muscle and vasodilation.
NO gas is catalyzed by the enzyme NO synthase from arginine. It passes readily across membranes and rapidly diffuses out of the cell into neighboring cells. NO has a very short half life (5-10 seconds) so It acts only locally. In many target cells, NO binds to iron in the active site of the enzyme guanylyl cyclase, stimulating this enzyme to produce cyclic GMP. The effects of NO can occur within seconds, because the normal rate of turnover of cyclic GMP is high. cGMP is rapidly degraded to GMP by a phosphodiesterase.
Increased cGMP activates a kinase that subsequently leads to the inhibition
of calcium influx into the smooth muscle cell, and decreased calcium-calmodulin
stimulation of myosin light chain kinase (MLCK). This in turn decreases the
phosphorylation of myosin light chains, thereby decreasing smooth muscle
tension development and causing vasodilation.
Other evidence suggests that cGMP works through an kinase (cGMP dependent
protein kinase PKG) that in turn phosphorylates a K+ channel to activate and
thus hyperpolarize the muscle cell
Nitroglycerine
Nitroglycerine, which has been used for about 100 years to treat patients with angina (pain resulting from inadequate blood flow to the heart muscle). The nitroglycerine is converted to NO, which relaxes blood vessels. This reduces the workload on the heart and reduces the oxygen levels needed by the heart muscle.
Viagra
The drug sildenafil [Viagra]inhibits this cyclic GMP phosphodiesterase and increases the amount of time that cyclic GMP levels remain elevated. The cyclic GMP keeps blood vessels relaxed and in certain parts of the male anatomy blood pools and the resulting effect has sales of Viagra soaring. It is interesting to note however that Viagra is not specific to the penis it will affect cGMP levels throughout the body and can have some interesting side effects.
Table 11-2. Effects of Acetylcholine Stimulation on Peripheral
Tissues
| Tissue | Effects of ACh |
| Vasculature (endothelial cells) | Release of endothelium-derived relaxing factor (nitric oxide) and vasodilation |
| Eye iris (pupillae sphincter muscle) | Contraction and miosis |
| Ciliary muscle | Contraction and accommodation of lens to near vision |
| Salivary glands and lacrimal glands | Secretion—thin and watery |
| Bronchi | Constriction, increased secretions |
| Heart | Bradycardia, decreased conduction (atrioventricular block at high doses), small negative inotropic action |
| Gastrointestinal tract | Increased tone, increased gastrointestinal secretions, relaxation at sphincters |
| Urinary bladder | Contraction of detrusor muscle, relaxation of the sphincter |
| Sweat glands | Diaphoresis |
| Reproductive tract, male | Erection |
| Uterus | Variable, dependent on hormone influence |
Putting it all together: From receptor to control of muscle cell contraction

Figure 21.7, From Molecular Biology of the Cell. Autonomic control of cardiovascular function.
The cardiovascular system is highly regulated so that there is always an
adequate supply of oxygenated blood to the body tissues under a wide range of
circumstances.
There are receptors that respond to the degree of blood pressure and provide
mechanical (barosensory) information about pressure in the arteries system
There are receptors that provide information about the level of oxygen and
carbon dioxide in the blood.
These sensory systems provide input to the respiratory control centers of the
brain which in turn control the parasympathetic and sympathetic nerves that
will control the heart, blood vessels and diaphragm muscles for breathing.
We will concentrate only on the chemoreceptors which are located primarily
in the carotid bodies. These are small, specialized organs located at the
bifurcation of the common carotid arteries (some chemosensory tissue is also
found in the aorta). The chemoreceptors in the carotid bodies and aorta
provide information about the partial pressure of oxygen (pO2) and carbon
dioxide (pCO2) in the blood.
This information is relayed by second order neurons to the hypothalamus and
other regions in the brainstem. This information about blood gas levels works
in a reflex to modulate the autonomic nervous system to control smooth and
cardiac muscles. It is a balance between regulation of the sympathetic versus
parasympathetic system to up or down regulate cardiac or smooth muscle
contraction.

From Cell Physiology, 2nd edition.
The carotid chemosensory cells detect levels of pO2 in the blood by simply
depolarizing in response to decreased levels of oxygen. The mechanism appears
to be an O2 sensitive K+ channel, that in the presence of normal levels of pO2
is open. Therefore the Vm is close to EK+. However a oxygen levels drop the K+
channel closes and Vm depolarizes allowing the voltage-gated Ca+2 channel to
open and to trigger vesicle fusion and neurotransmitter release.
It is thought that one way that this might occur is that the the O2 activates
the K+ channel by binding to a heme protein that is attached to the K+
channel.

From Cell Physiology, 2nd edition.
Conversely changing the degree of the K+ channel opening will allow those cells that spontaneous fire action potentials to increase their rate and thus signal a change in pO2 levels
 From Cell
Physiology, 2nd edition.
From Cell
Physiology, 2nd edition.
pO2 levels can have a direct effect on smooth muscles around blood vessels.
Many of these cells have K+ channel that is inhibited by ATP. As pO2 drops so
does respiration and ATP production. This reduction in ATP results in the
opening of K+ channels and the inhibition of smooth muscle contraction. This
results in the relaxation of the smooth muscles the relaxation of the blood
vessels and the increase blood flow into the tissue that is experiencing
reduced pO2.
Conversely an increase in pO2 results in greater inhibition of the ATP
sensitive K+ channels and thus a greater degree of depolarization. More Ca+2
channels are open and thus there is greater cytosolic Ca+2 levels, greater
degree of smooth muscle contraction. This causes the blood vessel to constrict
(vasoconstriction) and less pO2 transfer to the surrounding tissues.