Chapters
Lodish 4th edition: Chapter 21 pages 921 - 924
Moyes and Schulte: Chapter 3 page 81-84
Diffusion
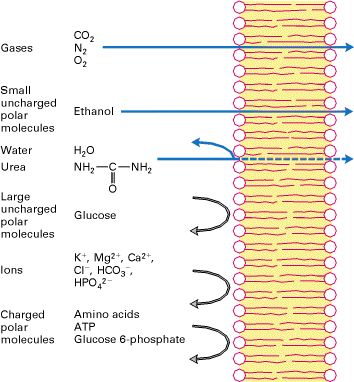
From Lodish, Molecular Cell Biology, 4th edition
All cells are encased in a lipid bilayer and therefore this poses a problem
for any charged molecules to cross the membrane. It is easy for gases to cross
the membrane and hydrophobic compounds readily cross. For instance ethanol and
heroin are examples of molecules that can cross the lipid bilayer without any
protein conduit. All other including water require a protein conduit to enable
them to get across.
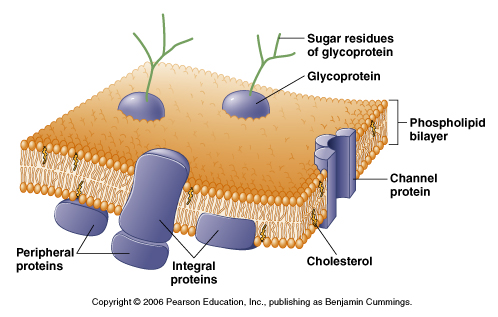
From Moyes, Animal Physiology
Membrane permeability and channels
The membrane proteins we will discuss in this course span the lipid bilayer
and allow for the flow of ions (channels) or the transport of molecules or
ions. Below are examples of some of the proteins we will be discussing:
From Lodish, Molecular Cell Biology, 4th edition
Each membrane protein contains a region of hydrophobic amino acids to generate
the transmembrane domain. This is essential to allow the protein to be
targetted to the membrane during protein synthesis and retain the protein in
the hydrophobic core of the lipid bilayer. Amino acids like phenylalanine (phe),
isoleucine (ile), leucine (leu) and alanine (ala) are commonly found in
transmembrane domains.
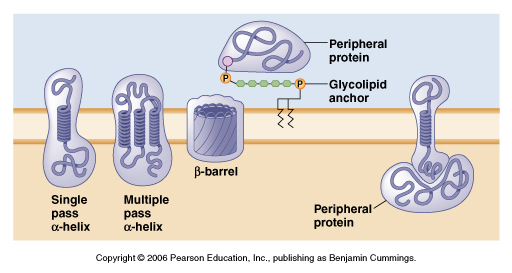
From Moyes, Animal Physiology
This poses a problem for ion channels or transporters which need to allow
charged molecules to traverse the membrane. For instance ion channels will
have a PORE or a region that spans the membrane containing charged amino
acids. This allows for ions such as K+ or Na+ to move from one side to the
other through the pore region. Each pore region is highly selective and will
only allow certain ions to flow across.
In these proteins the pore region is "protected" or isolated from the
hydrophobic environment of the lipid bilayer by a surrounding region of the
hydrophobic amino acids.
Membrane Transport Proteins
If essential molecules can not diffuse across the membrane then they must
be transported or have channels to allow passage.
There are many ways to bring hydrophilic or charged molecules across the
membrane, ion channels, ATP powered pumps or transporters.
Many of these transporters have been purified and cloned and can be studied in
isolation by expression of the clone in cells or reconstitution of the protein
in liposomes.
The function of channels and transporters can be tested by introducing the
protein into artificial lipid bilayers. When the protein is isolated the
ability to transport or allow ions to cross the membrane can be tested.
The following diagram shows the isolation of the glucose transporter in a
liposome generated from purified phospholipids.
From Lodish, Molecular Cell Biology, 4th edition
Facilitated Diffusion (Uniporter)
The easiest way to transport a molecule is down it's concentration
gradient. These uniporters transport molecules that are thermodynamically
favoured to enter the cell but can't because they are not able to diffuse
across the lipid bilayer. These molecules include amino acids, nucleosides,
sugars etc.
For this course we will discuss one uniporter, the glucose uniporter.
GLUT1 (mammalian glucose transporter)
Used by most mammalian cells to get glucose across the membrane.
Know a lot about its function and kinetics through studies that place the
protein in liposomes (see figure above).
This transporter (and all uniporters) use the concentration gradient of the
glucose to drive transport.
The transporter can work in reverse thus if the concentration of glucose is
higher on the inside can transport glucose out of the cell.
From Figure 15-5 you can see that the kinetics of transport can be thought of
in terms of the Michaelis-Menten equation where v = Vmax ([glucose]/[glucose]
+ Km).
From Figure 15-5 you can also see that without the transporter to facilitate
diffusion then the rate of entry of glucose into the cell almost zero.
There is a maximal rate of transport and the rate of transport is dependent on
the glucose concentration. Thus the Km can be calculated from the half maximal
rate. The Km for glucose is 1.5 mM. This reflects the affinity that the
transporter has for glucose. The lower the Km the greater the affinity. As we
will see with the Ca+2 ATPase it's internal Ca+2 binding sites have a Km =
0.0001 mM.
It is also clear that there is a favourable free energy for the transport of
glucose.
The concentration of glucose in the blood around 3.6 mM - 5.0 mM After glucose
is transported into the cell it is phosphorylated to form glucose 6-phosphate,
which cannot leave the cell. Because this reaction is the first step in the
metabolism of glucose which is rapidly used the favourable free energy of
glucose transport is maintained.
Nernst potential
All cells have a membrane potential (an electrical potential) that exists across the cell membrane. Researchers use microelectrodes to measure the voltage difference between the outside and inside of the cell. You can measure the membrane potential of a cell = the voltage difference between the inside and the outside of the cell.
Nernst equation:
Used to calculate the exact electrical potential at equilibrium that is
generated for a known concentration difference in a specific ion, separated by
a membrane permeable to that ion.
Walther Nernst (1888) derived this equation, based purely on theoretical
considerations.
The free energy associated with the transport of an ion (X) across the
membrane from the outside to the inside can be written out as:
DG = RTln([Xi]/[Xo]) +
zFEm
This is because there are no bonds broken or generated and no heat generated
so DG01 is zero.
As well because the ion is charged there is both a chemical component
RTln([Xi]/[Xo]) and an electrical
zFEm component.
At equilibrium then DG is zero and so:
zFE = - RTln([Xi]/[Xo])
Thus the equilibrium potential for ion X is:
Ex = - RT ln [X]i
...........zF.....[X]o
OR
Ex = RT ln [X]o
.........zF.....[X]i
R = universal gas constant, T = absolute temperature, z =valence of ion (i.e.
Cl- = -1), F = Faraday's constant
Note: the valence of the ion is very important to
remember!!
What does the equation mean in terms of two different ion concentrations
separated by a membrane?
Imagine two chambers separted by a membrane which is only permeable to
K+ and not to Cl-. The solutions on either side of the membrane
contain KCl.
Using electrodes measure the voltage (potential) difference across the
membrane when:
The concentration of KCl is equal on either side (0.01M) and so no there is no potential difference.
The membrane potential is: 0 mV
EK+ = RT/F ln(0.01/0.01) = 0 mV
Now increase the KCl concentration by 10 fold in chamber I
K+ flows down its concentration gradient, chamber II becomes more positively charged than I. The process reaches a point where no more K+ ions flow into II becaused balanced by equal flow of K+ ions out due to electrical repulsion - the system has reached an equilibrium.
The equilibrium between:
i) the chemical gradient which drives K+ into chamber II
ii) the electrical gradient which drives K+ out of chamber II
Therefore at equilibrium if one K+ enters II another K+ ion will be repelled - no net flux occurs.
We can use the Nernst equation to calculate what the membrane potential
will be at equilibrium.
EK+ = RT/zF (0.01/0.1) = -58 mV (at 22oC)
Each ion has a different potential given the difference in concentration
gradients.
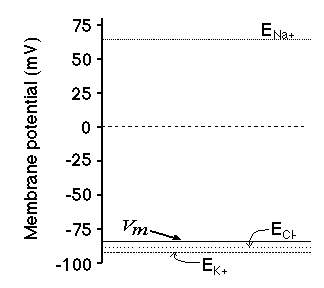
Remember the membrane has to be permeable to the ion. Ions can only cross the
membrane through pores or channels. If the membrane does not contain the
appropriate ion channel then no ion flow and no potential is created.
Chemical gradients in animal cells
These differences in Nernst potential reflect the differences in the
chemical gradients for each ion.
All animal cells maintain chemical gradients across their plasma membrane and
organelle membranes. As we will discuss there is large concentration gradient
of Ca+2 in all cells such that the cytosol has a very low Ca+2 concentration
while the outside of the cell and in the organelles such as the ER,
mitochondria Ca+2 is highly concentrated.
All animal cells also are characterized by a large K+ gradient so that the
inside of the cell has a higher K+ concentration than the outside. There is
more Na+ on the outside compared to the inside.
From Lodish, Molecular Cell Biology, 4th edition
We will concentrate on the protein pumps that are necessary to maintain these
gradients and more importantly why the cell would got to all this trouble to
use a large of energy to do this.
Free energy associated with the Na+ electrochemical gradient.
An example of the advantages of creating an electrical/chemical gradient
are outlined for Na+:
From Lodish, Molecular Cell Biology, 4th edition
The forces of the ion and the voltage gradients govern the movement of the
ions across the membrane. We can calculate the free-energy change (
DG) that corresponds to the transport of an ion
across the membrane.
Because ions are also charged the calculation included both a chemical and
electrical component.
For instance the the free-energy change generated by the Na+ ion concentration
gradient is:
DGc = RTln([Na+in]/[Na+out])
In our sample cell this corresponds to -1.45 kCal/mol (the change associated
with the transport of 1 mole of Na+ from the outside to the inside of the
cell).
The free-engery change generated by the membrane electrical potential is:
DGm = zFEm
where F = Faraday constant, Em is the membrane potential (-70 mV in most
animal cells) and z is the valence of the ion (+1 in this case). This would
correspond to -1.6 kCal/mol.
Because Na+ is affected by both the Na+ concentration gradient and the
membrane potential both are added to gether to give a total of -3.06 kCal/mol.
Therefore because this is less than 0 this favours thermodynamically the
movement of Na+ into the cell. This feature of Na+ we will see in different
examples in class can drive a number of cellular processes.