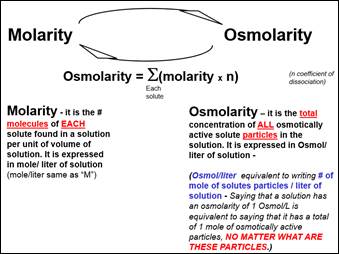
By using
this equation, we assume that we are working with IDEAL solutions. In ideal solutions,
solutes molecules dissociate in predicted fashions and solute particles do not
re-associate with each other once dissolved.
As you know, the
REAL world is usually quite different from the IDEAL one.
REAL solutions
do not quite behave like ideal solutions: for example, in real solutions, there is a
statistical probability that some cations and anions of a dissolved
electrolytes can re-associate temporarily at a given time.
This
difference between IDEAL and REAL solutions means that the true osmolarity of a REAL
solution is slightly different from its IDEAL equivalent calculated with the
equation above.
REAL SOLUTION: real (= measured) osmolarity – is measured with an osmometer
IDEAL
SOLUTION: ideal (= theoretical) osmolarity – is calculated with the equation above.
REAL
osmolarity / IDEAL osmolarity
= f
f is the osmotic coefficient of the solution
The osmotic coefficient (f) of a solution can be
determined from its colligative properties.
The
colligative properties of a solution are
- the properties that depend on the total number of solute particles in
a given volume, regardless of their chemical types;
- a solution osmotic pressure, depression of the freezing point, elevation of the boiling point and depression of the
water vapor pressure.
By comparing the
solution’s theoretical (= ideal) colligative properties with the real (=
measured) colligative properties, we can determine the osmotic coefficient of a
solution (f). For example:
the theoretical freezing point depression (∆Tf) of a solution can be calculated (Ideal ∆Tf) as well as measured (Real ∆Tf)
and f = (Real ∆Tf) / (Ideal ∆Tf)
The value of the osmotic coefficient of a solution
depends on many factors such as the concentration of
the solutes present; the types of solute presents; temperature, etc…
To illustrate, we have listed the
osmotic coefficients of solutions – Each solution listed contains only one type
of solute and the solute concentration is the same as what is found in the
extracellular fluids of mammals - the temperature of all the solutions is 37oC.
Solutions containing single solutes:
IDEAL
osmolarity = molarity x n
If
f is given to you, you can calculate their REAL osmolarity:
REAL
osmolarity = IDEAL osmolarity
x f
REAL
osmolarity = molarity x n x f
n: number of particles that dissociated from the solute molecule.
f:
osmotic coefficient of the solute (can be found on tables)
Solution containing several solutes:
IDEAL
OSMOLARITY = SUM OF ALL (molarity x n) OF EACH SOLUTE
(e.g.
for an ideal solution of 0.3M of glucose, 0.2M NaCl
and 0.1M CaCl2
the osmolarity is (0.3 x 1) + (0.2 x 2) + (0.1 x 3) =
1.0 Osm)
The REAL OSMOLARITY could be calculated using this equation:
REAL osmolarity = SUM OF ALL (molarity x n x f) OF EACH SOLUTE.
In practice it is quite impossible to determine f for each individual solute within this solution that is made of many type of solute. Therefore it is best to measure the REAL osmolarity using an osmometer.