Lecture 5 & 6
Regulation of the b-globin locus
Today we will be discussing the following paper:
Long-range chromatin regulatory interactions
in vivo.
Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P.
Laboratory of Chromatin and Gene Expression, Developmental
Genetics Programme, The Babraham Institute, Cambridge CB2 4AT, UK.
Nature Genetics 2002 Dec;32(4):623-6
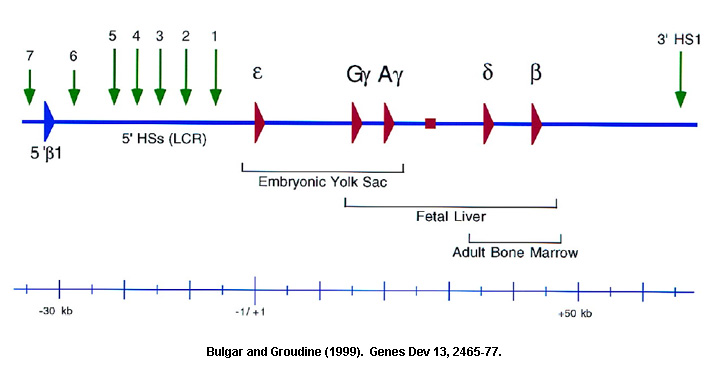
Introduction: The mammalian b-globin locus provides a complex but useful model for studying developmental gene regulation. The mouse and human b-globin genes (e, Gg, Ag, d and b) are clustered over a span of 50kb on chromosomes 7 and 11, respectively, and are arranged in the order of their expression during development.

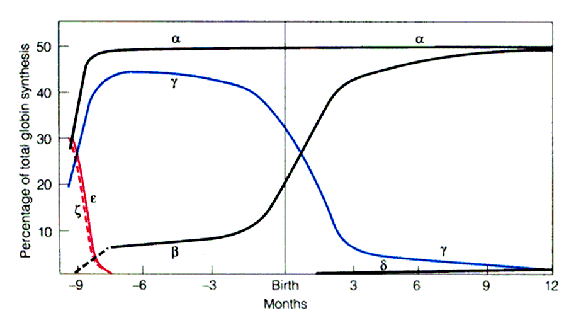
Both gene proximal and gene distal elements have been implicated in the tissue-specific and developmental regulation. The transcriptional silencing of the human b-globin genes by naturally occurring deletions in regions far upstream of the b-globin locus (b thalassemias) suggested that this region may be important in regulating the expression of the cis-linked genes. DNase I hypersensitive site (HS) mapping revealed five major HSs (5'HS 1–5) in this region, from 6 to 22 kb 5' of the human e-globin gene. The importance of these HSs was further suggested by their high degree of evolutionary conservation and the erythroid-specific formation of 5'HS 1–4 in human tissue. Fusion of DNA fragments containing 5' HS 1–5 to genes of the human b-globin locus led to high-level, position-independent expression in transgenic mice. This contrasted with earlier studies in which isolated b-globin transgenes demonstrated extensive variation in expression. The region containing 5'HS 1–5 was designated the locus control region (LCR) and was defined operationally by its ability to enhance the expression of linked genes to physiological levels in a tissue-specific and copy number-dependent manner at ectopic chromatin sites in the genome. The sequence elements that are found at individual HSs consist of multiple arrays of ubiquitous and lineage-specific transcription factor binding sites.
Locus Control Region (LCR):
The high level of expression driven by the LCR at ectopic sites suggested that gene regulation by the LCR involved at least two activities: the transcription-enhancing activity and the formation of an open or active chromatin domain within which such activation can take place. However, when the entire native mouse LCR was deleted in cell lines, the formation of the general DNAse I sensitivity associated with the b-globin locus domain was not affected (Bender et al. 2000. Molecular Cell 5, 387-93). The transcription rates were dramatically reduced. Thus, it has been proposed that the dominant role of the LCR is to confer high-level transcription, and elements elsewhere in the locus are sufficient to establish and maintain an open conformation and to confer developmentally regulated globin gene expression. This is in contrast to transgenic analyses that show a requirement of the LCR for the establishment and maintenance of an open b-globin chromatin domain and transcriptional activation. It remains to be seen how these data will be reconciled.
How does the LCR accomplish transcriptional activation of the b-globin genes? Several models have been proposed and are shown in the figure below.

Models of LCR function. A globin gene is denoted by a green rectangular box with the promoter region indicated in a lighter green. Transcription factors are shown as colored ovals and circles. The 4 erythroid-specific hypersensitive site cores (HSs) are indicated by small red boxes. Blue boxes are the positions of 5'HS5 and 3'HS1, representing likely insulator elements. The flanking DNA sequences of the HSs are depicted as loops between the HS cores. Transcripts are denoted by wavy arrows. (A) Looping model. Transcription factors bind to the LCR HSs and the gene promoter. The LCR directly interacts with the gene promoter by looping out the intervening DNA, thus forming an active transcription complex at the gene promoter. (B) Tracking model. Sequence-specific transcription factors bind to the LCR, forming a complex that tracks down the DNA sequence, as depicted by the large black arrowhead, until encountering transcription factors bound to the appropriate gene promoter, initiating high-level gene expression. (C) Facilitated tracking model. Aspects of both looping and tracking models are combined. Sequence-specific transcription factors bind the LCR; looping then occurs to deliver the bound transcription factors proximal to the gene promoter, followed by tracking, until they encounter transcription factors bound to the appropriate gene promoter. (D) Linking model. Sequential binding of transcription factors along the DNA directs changes in chromatin conformation and defines the transcriptional domain. The transcription factors are linked to one another from the LCR to the gene promoter by non-DNA-binding proteins and chromatin modifiers (shown as small colored circles). From Blood 100, 3077-3086 (2002).
The first three models of LCR function (A-C) fall into the contact model proposed for how enhancers work. The contact model proposes that communication between enhancers and promoters occurs through direct interaction between the distant enhancer and the promoter it is activating/silencing. This direct contact is facilitated by various mechanisms that 'loop out' the intervening sequences. The last model (D) falls into the non-contact model. This model proposes that enhancer action involves indirect effects exclusively that initiate or nucleate at the enhancer and are subsequently transmitted along the chromatin fiber to linked genes.
The paper we will be discussing today uses
the b-globin locus as a model system to study
enhancer-promoter interactions. They have used a novel combination of FISH
and ChIP techniques to provide new insight into the mechanism by which enhancer
elements exert their control.
Long-range chromatin regulatory interactions
in vivo.
Carter D, Chakalova L, Osborne CS, Dai YF, Fraser P.
Laboratory of Chromatin and Gene Expression, Developmental
Genetics Programme, The Babraham Institute, Cambridge CB2 4AT, UK.
Nature Genetics 2002 Dec;32(4):623-6
Figure 1. RNA TRAP
Figure 2. Measurement of the spread of biotin deposition.
Figure 3. Quantitative real-time PCR analyses of Hbb-b1 targeted RNA TRAP.
Figure 4. Quantitative real-time PCR analyses of Hbb-b2 targeted RNA TRAP.
Figure 5. Schematic representation of LCR-gene arrangement.
Discussion points:
1. The RNA TRAP data indicates that the region of DNA within the LCR corresponding to HS2, is in close proximity to the actively transcribed Hbb genes relative to other sequences.
2. This provides strong evidence for looping models of enhancer function.
3. Enrichment of the Hbb promoters is slightly lower than enrichment of HS2 suggesting that HS2 is closer to the nascents mRNA transcripts than to the promoters (figure 4). Can the LCR be involved in transcription elongation rather than initiation?
4. Comparing figures 3a and 4 you will notice a second peak of enrichment over HS4 of the LCR in the RNA TRAP experiment performed with oligonucleotides specific for Hbb-b2. This is absent when performed with with probes specific for Hbb-b1. Previous analyses have shown that deletion of most HS sites, including HS4 and HS2, results in a greater decrease in expression of Hbb-b2 than of Hbb-b1 (Alami, R. et al. 2000. Genomics 63, 417-424). Does this suggest that particular HS - promoter interactions exist?
5. Targeted deletion of HS2 does not completely abolish transcription from the Hbb promoter (Fiering et al. 1995. Genes Dev 9, 2203-13). In fact, Hbb transcription levels are only reduced 40%. If the proximity of HS2 to the Hbb transcripts were critical for gene activation, wouldn't you expect a more severe reduction in the rate of transcription?
6. Do the biotinyl–tyramide radicals produced react preferentially with particular amino acids or do they have equivalent reactivity? If the former, proteins containing these amino acids will be the primary targets of the RNA TRAP technique. Thus, if particular sequences are bound by more reactive proteins than others, these will be preferentially labeled.
7. Future experiment: Corroborate the main findings via different assay systems, providing that they don't suffer from the same limitations. DNA TRAP? It has yet to be developed but has been suggested. Perhaps EM analysis using reconstituted protein complexes and DNA sequence in vitro.