Lecture 13
Drosophila Development
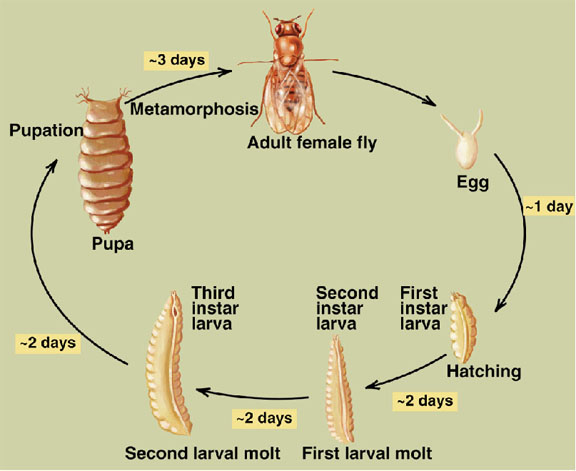
In order to discuss gene regulation in development, I want to tell you enough about the life cycle, embryology and developmental genetics of early Drosophila melanogaster. You need to know the basics in order to understand the context of the work on gene regulation that we are going to concentrate on for the rest of the course. The information in this lecture is by no means comprehensive as this is a course in gene regulation rather than developmental genetics.

1. Embryology of Drosophila
Oogenesis: In
many animals, development starts by the formation of the egg inside the mother,
a process known as oogenesis
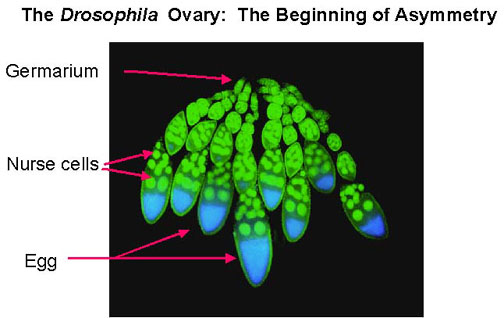
Drosophila ovaries consist of many finger-like projections called ovarioles. Each ovariole is an independent egg assembly and has a range of oocytes from very young to mature, together with a collection of nurse cells that deposit proteins and mRNA into the oocyte. Each ovariole begins with a structure called a germarium, where germ cells and somatic cells are organized into egg chambers. Egg chambers leave the germarium and continue developing as they move posteriorly in the ovariole. As each egg is laid, egg chambers move down the ovariole, so one can see a complete chain of oocyte development from beginning to end.
In the Drosophila ovary, each oogonium (primordial female germ line cell) undergoes four incomplete mitotic divisions, to produce 16 cells all connected by cytoplasmic bridges. One of these becomes the oocyte, and the rest serve as nurse cells, which supply cytoplasmic macromolecular components to the oocyte as it grows and matures. This is known as meroistic oogenesis and differs from panoistic oogenesis where all oogonia develop into oocytes. Somatic follicle cells form an epithelial layer around each oocyte and accompanying nurse cells; they are connected to the oocyte by gap junctions, which allow exchange of small molecules only. They also may secrete macromolecules that interact with the oocyte cell surface or are taken up by endocytosis.
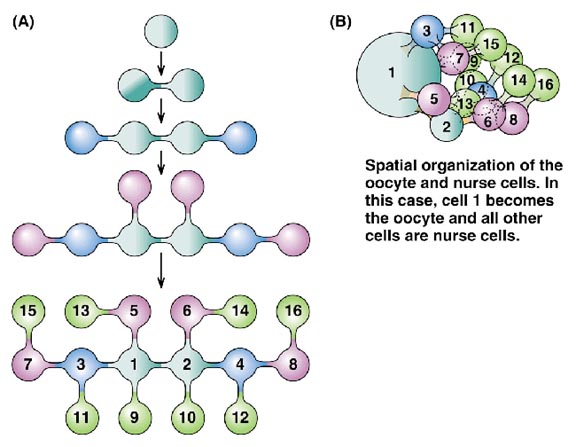
As illustrated above, the oocyte is associated with a cluster of nurse cells which are invariably located on one side of the oocyte, facing the germarium at the tip of the ovariole. This side becomes the anterior pole of the egg, where the head of the embryo will be formed. The events leading from the arrangement of the nurse cell/oocyte in the egg chamber to the anterior-posterior body pattern of the embryo have been elucidated in large part by genetic analysis.
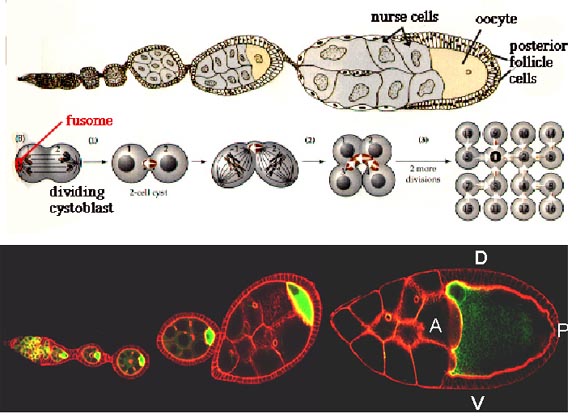
One of the key events in assigning polarity to the embryo is the transcription of bicoid mRNA in the nurse cells followed by its deposition into the oocyte. Upon entry into the oocyte, bicoid mRNA becomes localized to the anterior pole presumably by attaching to cytoskeletal components. When the localized bicoid mRNA is translated, bicoid protein forms a morphogen gradient, with a maximum concentration at this pole. Visit http://www.luc.edu/depts/biology/dev/bicoid.htm for an animation of this process. The highest concentration ranges of this morphogen activate embryonic genes involved in the head and thorax development.
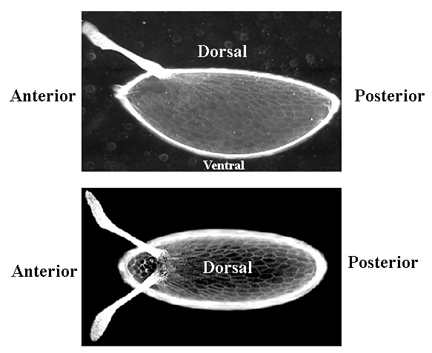
The unfertilized egg has a very obvious polarity: the future dorsal surface is flat and the ventral surface is rounded. The anterior end is pointed whereas the posterior end is rounded.
Early embryogenesis: Drosophila eggs have a great deal of yolk at the center of the egg. The first 7 nuclear divisions occur in the center of the egg, and then most nuclei migrate out to the periphery. There is no cell division until cell cycle 14, so the early Drosophila embryo is a syncitium. This means that molecules can freely diffuse. The first cell divisions are very rapid, as the first occurs 20 minutes after fertilization and the subsequent divisions follow at 10 minute intervals. Each of the first 13 divisions is synchronous.
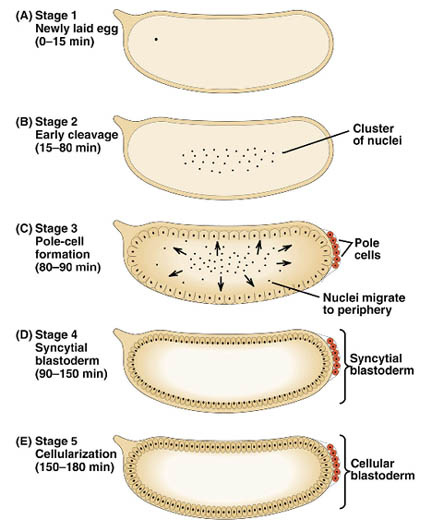
The stage occupying cell cycle 10-14 is called the syncitial blastoderm, and is the period when most of the nuclei are at the periphery, until the first appearance of cell membranes. It begins about 90 minutes after fertilization and lasts for 2 hours. Cell cylce 10 marks the first sign of transcription from the zygotic genome. Cell cycle 14 marks cellular blastoderm and occurs at 2.5 to 3 hours after fertilization.
Gastrulation: Gastrulation, the process of reorganizing the embryo to form 3 primary germ layers, begins immediately after establishment of cellular blastoderm. The first sign is the invagination of a midventral band of cells to form the ventral furrow. The invaginating cells will form the presumptive mesoderm. The cells at the boundaries of the invagination will form neuroblasts that will eventually give rise to the CNS.
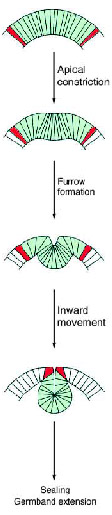
During the formation of the mesoderm, a transverse groove, called the cephalic furrow forms at the anterior border of the ventral furrow. This is a zone of ingression of cells that will go on to form the anterior foregut, called the anterior midgut invagination. At the posterior end, a similar invagination forms, called the posterior midgut invagination. Eventually these two invaginations will meet in the middle, to form the endodermal gut.
Immediately proceeding gastrulation is germ band extension. The first visible signs of segmentation occur at this stage. At 9 hours after fertilization, the germ band retracts so the body parts end up in the conventional anteroposterior order.
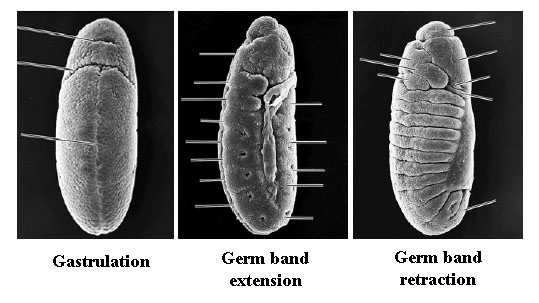
2. Segmentation
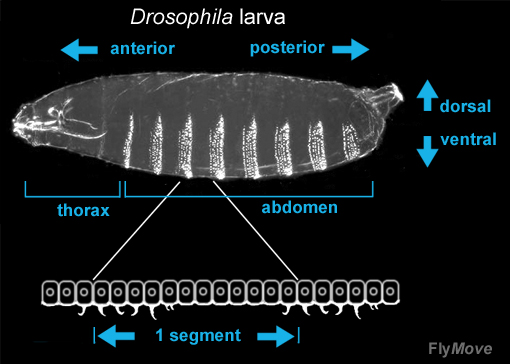
A common characteristic of embryos of many higher organisms is the formation of repeated, morphological units called segments or metameres. Drosophila melanogaster is segmented in two distinct registers: the embryonic parasegmental register and the larval/adult segmental register. Parasegments are embryonic metameric units which include the primordial cells of the posterior part of one post-embryonic segment.
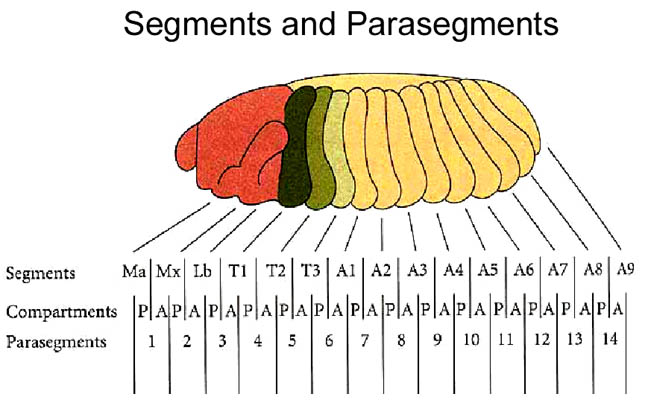
The Drosophila larva bears characteristic rows of denticles at its ventral side. Segments in different parts of the body show characteristic denticle patterning, and within each segment, single cell rows can be identified through presence or absence, as well as characteristic shape of the denticles.