Protein Processing I: Getting things where
they need to go -Targeting of Proteins and Vesicles
The key concept:
Proteins are synthesized in the cytosol compartment
and are targeted to many sites in the cell: mitochondria, chloroplasts, endoplasmic
reticulum, nuclei etc. Vesicles that bud off of the plasma membrane, the endoplasmic
reticulum or the Golgi apparatus are also targeted to specific sites in the
cell. How does this happen?. The basic idea is very similar to that underlying
the postal service:
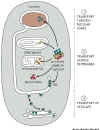
Figure 14-5
|
- Each protein contains information in amino acid sequences
that serve as an address. All proteins targeted to the same destination,
e.g. nucleus, carry the same address signal encoded in their protein
sequence.
- There is a specific protein receptor
that corresponds to each type of targeting signal. It binds only
to the correct targeting signal sequence. This second part of the system
corresponds to the street addresses on buildings in a city.
|
Protein Targeting
To nuclei
The nuclear localization signal (NLS) is a sequence of several
amino acids, Pro-Pro-Lys-Lys-Lys-Arg-Lys -Val-,
containing a run of basic amino acids that is located within a protein sequence,
rather than at the ends.
- This sequence is exposed somewhere
on the surface of the protein.
- It combines with a nuclear
import receptor protein.
- The complex of the nuclear import
receptor and the protein to be imported then binds to the fibrils that extend
on the cytosol side of the nuclear pore complex. This interaction is specific
and depends on the nuclear import receptor being bound to the NLS protein.
- The nuclear pore apparatus then transfers the nuclear import receptor-NLS
protein complex into the nucleus. This step requires energy (GTP).
- The nuclear import receptor then
detaches from the NLS protein, and the nuclear import receptor proteins return
to the cytosol and are reused.
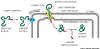
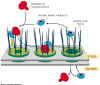 |
Figure 14-9 Import of proteins
into the nucleus. Notice the role of the nuclear import receptor protein
(blue). Link to animation |
To or across membranes
Proteins targeted to or across membranes typically
carry a signal sequence at the N terminal end of the protein.
To Mitochondria, Chloroplasts or Peroxisomes
 |
Key concept: The protein containing the proper
signal sequence binds to a receptor , bound. It is then unfolded as it traverses
the membrane through a protein channel, and is refolded on the organellar
side of the membrane. See Fig 14-10 (left). This requires a set of special
organellar chaperone proteins. |
- For mitochondria the signal sequence is: +H3N-Met-Leu-Ser-Leu-Arg-Gln-Ser-Ile-Arg-Phe-Phe-Lys-Pro-Ala-Thr-Arg-
Thr-Leu-Cys-Ser-Ser-Arg-Tyr-Leu-Leu-
- The protein containing the signal sequence
is synthesized in the cytoplasm.
- Signal sequence binds to a receptor in
the organelle membrane
- Receptor - protein complex diffuses within
membrane to a contact site.
- Protein is unfolded, moved across the membrane,
and refolded. These operations are carried out by the protein transporter
complex and its associated chaperone
proteins. Remember chaperones from earlier discussion
of protein processing? The signal sequence is the first part of the
protein to enter the organelle.
- Once inside, the signal sequence is
cleaved off by a specific peptidase.
To endoplasmic reticulum.
Synopsis. Synthesis of proteins entering
the endoplasmic reticulum is initiated on free ribosomes. A targeting sequence
of hydrophobic amino acids near the amino terminal end of the growing
polypeptide results in the binding of the ribosome to ER membrane and in insertion
of the polypeptide into the endoplasmic reticuluum.
Proteins going to Golgi, endosomes, lysosomes
and ER all enter the ER and don't come out again.
There are two groups of proteins targeted
to the ER:
- Proteins that are completely translocated
into the endoplasmic reticuluum. These proteins are soluble (not membrane
proteins) and are destined for secretion, or for the lumen of another organelle.
In all of these cases the proteins are never part of membranes.
- Proteins that are inserted into membranes,
and hence are only partially translocated into the endoplasmic reticuluum.
These proteins may be destined for ER, another organelle, or the plasma membrane.
In all of these cases the proteins stay within the membrane (e.g.
cellulose synthase).
Let's deal first with the case of proteins
that will be inserted into the ER lumen:
- The signal sequence is located at the N terminal end of the protein: +H3N-Met-Met-Ser-Phe-Val-Ser-Leu-Leu-Leu-Val-Gly-Ile-Leu-Phe-Trp-Ala
Thr-Glu-Ala-Glu-Gln-Leu-Thr-Lys-Cys-Glu-Val-Phe-Gln-
- The long sequence of about 10 hydrophobic amino acid residues (in blue,
above) is a membrane crossing domain.
- Targeting to the endoplasmic reticulum takes place through the interaction
of the signal peptide sequence ( a sequence of at least eight
hydrophobic amino acids at the amino terminal end of the polypeptide. The
emerging signal sequence combines with a 'signal recognition particle'
(SRP). This greatly reduces the rate of translocation and allows the ribosome
to attach to the endoplasm reticulum by means of a special SRP receptor
in the ER membrane.
- The ribosome becomes attached to a ribosome receptor that
also functions as the translocation channel for the newly
synthesized polypeptide. As the ribosome becomes attached, the SRP is removed
and translation resumes.
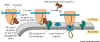 Figure 14-13. shows two components:
Figure 14-13. shows two components:
- There is a Signal Recognition Particle (SRP) in the cytosol. This binds
to the ER Signal sequence when it is exposed on the ribosome and slows
protein synthesis long enough to allow the SRP to find the second part,
the SRP Receptor.
- The Signal Recognition Particle Receptor (SRPR) which is embedded in
the ER membrane. We now have the new polypeptide synthesizing system in
place and protein synthesis speeds up. It seems that the Signal Sequence
opens the translocation channel.
- After translation is complete, the signal sequence, which is embedded into
the ER membrane, is cleaved off of the protein by a specific signal peptidase,
an enzyme that is present in the ER lumen. This leave the newly synthesized
protein free in the lumen of the ER. See animation
of this process
Proteins inserted into membranes:
- There are three types of hydrophobic signals
used in insertion of membrane proteins. All of these are membrane crossing
domains:
- Signal peptide sequence - a
cluster of about 8 -10 hydrophobic amino acids at the N-terminal end of
a protein. This sequence remains in the membrane and is cleaved off of
the protein after transfer through the membrane. This is the same
as the signal peptide sequence mentioned above.
- Start transfer sequence. Similar
to a signal sequence, but located internally (not at the N terminal end
of the protein). It also binds to the SRP and initiates transfer. Unlike
the N-terminal signal sequence, it is not cleaved after transfer of
the protein.
- Stop transfer signal. This is
also a sequence of about 8 hydrophobic amino acid residues. It follows
either a N-terminal signal sequence or a start transfer sequence.
- The stop transfer signal is a
membrane crossing domain. It remains in the membrane.
- When it is is encountered the translocation
channel is disassembled.
- The peptide is not cleaved.
- Translation continues in the cytoplasm.
- If a subsequent Start Transfer
sequence is encountered in the protein, a second SRP binds to the
start transfer sequence and a new translocation channel is opened
in the membrane.
The process works as follows:
Whenever a N terminal signal sequence or a
start transfer sequence is produced by the translation process, it bind to a
signal recognition particle that results in attachment to the ER and the formation
of a translocation channel. If a stop transfer sequence is encountered, translocation
is stopped, although translation continues to the end of the molecule. If a
subsequent start transfer signal is encountered a new SRP binds and a new translocation
channel is formed.
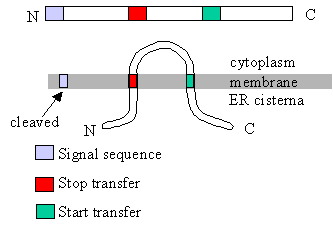 |
This diagram shows the relation
between translocation control sequences (signal sequences, start transfer
sequences, stop transfer sequences) and the arrangement of the protein in
the membrane. How would the translocation control sequences have to be arranged
to get the N terminal end of the protein on the cytoplasmic side?
- to get the C terminal end of the protein on the cytoplasmic side? Animations
for insertion of membrane proteins (including multi-pass proteins). |
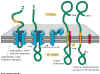
Figure 14-15. Click to enlarge
Animation
of this process
|
Import of a membrane protein
into the membrane of the ER. The blue sheath-like component shown in the
figure is the translocation channel that moves the protein through the membrane.
Notice that the Stop Transfer sequence (orange) results in the disassembly
of the translocation channel. Note that the signal sequence at the N terminal
end of the protein is cleaved off, releasing the N terminal portion of the
protein into the ER lumen. This example is a single pass membrane
protein that contains a single membrane crossing domain (the stop transfer
signal). |
Vesicle Formation and Targeting

Fig. 14-17 Vesicle traffic in cells. Click to enlarge. |
Individual protein molecules
are targeted to various destinations within the cell. Individual proteins
are not the only things moving in cells. There is a tremendous flux
of vesicles within most cell types. Vesicles form from the endoplasmic reticulum,
the Golgi apparatus and the plasma membrane. They are used to transport
membrane and proteins between many different membranous organelles.
Here we will be looking at how vesicles are formed and how they find their
targets. |
Vesicle formation and vesicle transport
Transport between these compartments takes
place via vesicles. Vesicle transport is the means by which membrane transport
occurs between compartments within the cell. Membrane proteins and soluble proteins
contained within the vesicles are also transported.
For example, once the proteins are in the
ER, they are transported by vesicles which bud off of the ER and
fuse with the membrane of the target compartment.
Vesicle transport presents substantial targeting
problems: each vesicle must take correct cargo to correct target.
The key to this form of transport
lies in the vesicle coats.
There are several types of coats but all have
two functions:
- to shape the membrane into a bud
- to capture molecules for onward transport.
See animation.
Each bud has a distinctive coat protein on
cytosol surface.
- The coat protein must shape the membrane
into a bud.
- The bud must capture the correct molecules
for outward transport
- After budding, the protein coat is lost.
- Bud can now interact with target - target
interaction signals are now exposed.
Two examples of target protein systems are
COP-coated vesicles involved in transport of vesicles from ER to Golgi and within
Golgi. and clathrin coated vesicles that carry proteins from the Golgi
to endosomes or from the plasma membrane to endosomes.
The best studied vesicles coated by proteins
are coated with a set of proteins called clathrins.
- Clathrin coated vesicles bud from the outer
(trans) face of the Golgi complex or from the plasma membrane..
- Clathrin coated vesicles are involved in
transport of materials by vesicles from the Golgi to the plasma membrane (exocytosis),
as well as transport from the plasma membrane to endosomes (endocytosis) .
To form a bud and initiate the budding process
you need to have stuff (cargo) in the package (vesicle) and then have to pinch
it off.
|
 Figure 14-19. Formation of coated vesicles.
Figure 14-19. Formation of coated vesicles.
|
This figure shows the process
of clathrin coated vesicle formation at the cell surface. The same process
occurs at the trans-Golgi to form vesicles that move toward the plasma membrane.
See animation. |
 Fig
14-18 Click to enlarge. Fig
14-18 Click to enlarge. |
Formation of a clathrin coated
vesicle. Notice the thickness of the cargo material attached to the cargo
receptors that extend through the membrane. The clathrins form a layer in
the cytosol side of the membrane, and are very important for selection of
cargo protein for transport. See animation. |
This process requires the interaction of several
components: cargo receptor, adaptin, clathrin and dynamin.
- The cargo molecule is picked up by the
cargo receptor, which is an integral membrane protein.
- The cargo receptor/cargo complex is recognized
by adaptin which combines with the cytosolic side of the cargo receptor molecule.
- The cargo/ cargo receptor/ adaptin complex
then combines with clathrin on the cytosolic surface.
- Clathrin forms the curved bud membrane
configuration
- Dynamin constricts the neck of the bud
(vesicle), which then pinches off.
- Uncoating then occurs as the clathrin and
adaptin are released and recycled.
- Each vesicle also has a specific targeting
signal as described below.
The vesicle is now ready for transport.
Vesicle targeting
Over short distances, movement of vesicles
is by diffusion. Transport of vesicles over longer distances is dependent on
cytoskeleton-based motor proteins.
Docking must be specific (don't know how it
works). For example, hemicellulose going to the plant cell wall is delivered
to sites where cellulose synthesis is occurring. Complementary fit is part of
the story, but snares are also involved.
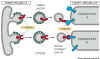
Figure 14-20. Snares and specificity of vesicle transport. |
Snares are proteins
that result is specific attachment of vesicles to their target membranes.
Snares occur as complementary pairs
of proteins. The vesicle-snare (v-snare) is incorporated into the vesicle
membrane, and the target-snare (t-snare) is incorporated into the
target membrane.
Docking occurs by interaction of the
v-snare and t-snare proteins. This binding is very specific.
|
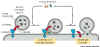
Figure 14-21. Transport vesicle fusion. |
Once the vesicle and the
target membranes are docked, several other proteins join to form a 'fusion
complex' that results in the fusion of the vesicle with the target membrane.
Fig 4-21. Following the docking of a transport vesicle at its target membrane,
a complex of membrane fusion proteins assembles at the docking site and
catalyses the fusion of the vesicle with the target membrane. |






