Chapters
Lodish 4th edition: Chapter 21 pages
Moyes and Shulte: Chapter 5 pages 186-193
Postsynaptic Membranes - Ligand gated ion channels
Figure 21-8, Lodish 4th adition. Ion channels in neuronal plasma membranes. Each type of channel protein has a specific function in the electrical activity of neurons. (a) Resting K+ channels are responsible for generating the resting potential across the membrane. (b) Voltage- gated channels are responsible for propagating action potentials along the axonal membrane. (c, d) Two types of ion channels in dendrites and cell bodies are responsible for generating electric signals in postsynaptic cells. One type (c) has a site for binding a specific extracellular neurotransmitter (blue circle). The other type (d) is coupled to a neurotransmitter receptor via a G protein; it responds to intracellular signals (red circle) induced by binding of neurotransmitter to a separate receptor protein (not shown). Signals activating different channels include Ca2+, cyclic GMP, and the Galpha subunits of trimeric G proteins
Most of the proteins that make up the different types of ion channels are
very similar in their structure and have conserved amino acid sequences. This
degree of conservation occurs between different types of channels and across
species. So for instance the Drosophila voltage-gated Na+ channel is very
similar to the human voltage-gated Na+ channel etc. The acetylcholine receptor
in C. elegans is very similar to the acetylcholine receptor in rabbets.
All the ion channels are composed of alpha helices that span the lipid bilayer.
Those that contact the lipid bilayer are composed of hydrophobic amino acids (Phe,
Ile, Leu etc.) that span about 20 amino acids. Those alpha helices that line
the pore are composed of hydrophilic residues to allow ion flow (Lys, Arg
etc.).
All the ion channels in question have a common feature. A pore that allows the ion(s) in question to flow across the lipid bilayer. The pore is specific to a certain ion or ions. For instance the leak K+ channel only allows K+ ions to flow across the membrane. The acetylcholine receptor allows Na+ to flow and the glycine receptor allow Cl- to flow through the channel.
Ligand gated ion channels are different than voltage-gated ion channels in
that they are chemically gated. They bind a small chemical that triggers the
opening of the ion channel
Ligand gated:
i) Na+ channels - excitatory (generates an excitatory postsynaptic potential)
ii) Cl- channels - inhibitory (generates an inhibitory postsynaptic potential)
The specificity of a transmitter response is a function of the receptor
type NOT the transmitter itself. (i.e. Ach can be excitatory when binding to
one type of AchR (NMJ)) and inhibitory when binding to another type of
receptor
The following (Table 21-1) list some ligand gated ion channels and the ions
they are permeable to.
Table 21
Neuromuscular junction
We will talk a lot about the Neuromuscular junction or synapse (NMJ) in this course. This is the synapse that occurs between a motor neuron and a muscle fiber. Later in the course we will see how the muscle fiber contracts but first we will discuss how this chemical synapse works.
Figure 21-33, Lodish 4th edition. Longitudinal section through a frog nerve-muscle synapse (neuromuscular junction). Synaptic vesicles in the axon terminal are clustered near the region where exocytosis occurs. The basal lamina lies in the synaptic cleft separating the neuron from the muscle membrane, which is extensively folded. Acetylcholine receptors are concentrated in the postsynaptic muscle membrane at the top and part way down the sides of the folds in the membrane. A Schwann cell surrounds the axon terminal.
Neurotransmitter of the NMJ
Acetycholine is the neurotransmitter at the NMJ. (Exception is glutamate in insects).
Acetycholine
- motor neuron transmitter at the neuromusccular junction (NMJ) in vertebrates
- present in brain (10% of synapses)
- packaged in high numbers in vescicles 1,000 to 10,000 molecules per vesicle
at the NMJ
- like all small chemical transmitters Ach is synthesized and packaged into
vesicles in the synpase
- the NMJ presynaptic side is packed full of vesicles in the axon terminal
(see Fig. 21-33 above)
- many vesicles are released per action potential to ensure a large safety
margin so that the muscle fiber (i.e. the postsynaptic cell) will depolarize
to beyond threshold.
Acetylcholine ligand-gated receptor:
- officially called the nicotinic nAChR because nicotine binds to this
receptor and activates it. - ligand gated ion channel
- generates an excitatory postsynaptic potential which at the NMJ is often
called an "end plate potential"
- has a depolarizing effect because Na+ is the dominant ion through these
channels at resting potentials. (Also allows K+ to flow but at rest K+ only
has a small influence. The reason behind this is a topic covered in Biology
455)
Figure 21-37, Lodish 4th edition. Figure 7-44, Lodish 5th edition. Sequential activation of gated ion channels at a neuromuscular junction.
Arrival of an action potential at the terminus of a presynaptic motor neuron induces opening of voltage-gated Ca2+ channels (step 1) and subsequent release of acetylcholine, which triggers opening of the ligand-gated nicotinic receptors in the muscle plasma membrane (step 2). The resulting influx of Na+ produces a localized depolarization of the membrane, leading to opening of voltage-gated Na+ channels and generation of an action potential (step 3).
EPP - end plate potential
Aka Excitatory Junctional Potential (EJP)
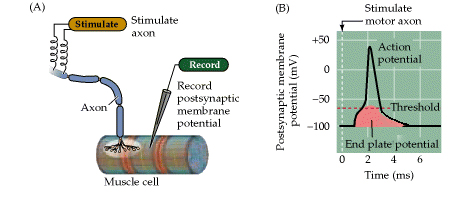
Figure 5.6. From Purves, Neuroscience, 3rd edition. Synaptic transmission at the neuromuscular junction.
(A) Experimental arrangement, typically using the muscle of a frog or rat. The axon of the motor neuron innervating the muscle fiber is stimulated with an extracellular electrode, while an intracellular microelectrode is inserted into the postsynaptic muscle cell to record its electrical responses. (B) End plate potentials (EPPs) evoked by stimulation of a motor neuron are normally above threshold and therefore produce an action potential in the postsynaptic muscle cell.
Structures of nAchR
Figure 21-38, Lodish 4th edition. Figure 7-45, Lodisth 5th edition. Three-dimensional structure of the nicotinic acetylcholine receptor based on amino acid sequence data, computer-generated averaging of high-resolution electron micrographs, and information from site-specific mutations.
(a) Schematic cutaway model of the pentameric receptor in the membrane; for clarity, one subunit is not shown. Most of the mass of the protein protrudes from the outer (synaptic) surface of the plasma membrane. The M2 alpha helix (red) in each subunit is part of the lining of the ion channel. Aspartate and glutamate side chains at both ends of each M2 helix form two rings of negative charges that help exclude anions from and attract cations to the channel. The gate, which is opened by binding of acetylcholine, lies within the pore. Inset: Cross section of the exoplasmic face of the receptor showing the arrangement of subunits around the central pore. The two acetylcholine-binding sites are at the interfaces of the alpha subunits; they are located about 3 nm from the membrane surface. (b) A view from above, looking into the synaptic entrance of the channel. The tunnel-like entrance narrows abruptly after a length of about 6 nm.
Synapses in the brain or central nervous system (CNS)
Figure 21-3, Lodish 4th edition. Figure 7-47, Lodish 5th edition. Typical interneurons from the hippocampal region of the brain makes about a thousand synapses.
The cells were stained with two fluorescent antibodies: one specific for the microtubule-associated protein MAP2 (green), which is found only in dendrites and cell bodies, and the other specific for synaptotagmin (orange-red), a protein found in presynaptic axon terminals. Thus the orange-red dots indicate presynaptic axon terminals from neurons that are not visible in this field.
- a single synapse on a target is seldom found in brain
- large neurons in the brain typically receive many inputs (1000 to 80,000 per
cell)
- the inputs are integrated in the receiving neuron such that a "decision" is
made to pass on the information onto other cells
- this "decision" is often whether or not to generate an action potential
- each synaptic input usually only gives only a small depolarization so many
imputs must cooperate (summate) to reach threshold to fire an
action potential
- for example in the case of the motorneuron to get an epsp of +20 mV would
need in the order of 20 terminals to simultaneously discharge (process called
summation)
EPSP
- an excitatory impulse, an excitatory post-synaptic potential raises the
membrane potential above rest
i) an excitatory impulse at a synapse on the soma causes a depolarization of
the whole soma including the beginning of the axon. This is because the
diameter of the soma or cell body is so large that the internal resistance is
very low so current flow extremely well through the cell body.
- the beginning of the axon is also known as the spike initialization zone or
axon hillock and is packed with Na+ channels, an epsp of +15 to +20 mV
triggers an action potential in the zone
ii) an epsp generated on a dendrite will diminish in strength by the time the
current has reached the soma such that an epsp in a dendrite has less of a
chance of triggering an action potential than an epsp generated at the soma
due to the absence of voltage-gated Na+ channels in the soma and dendrite of
most neurons it is very unlikely that an action potential will be generated in
these regions
IPSP
- an inhibitory impulse is called an i.p.s.p (inhibitory post-synaptic
potential) and lowers the membrane potential below rest (hyperpolarizes)
- synaptic transmission triggers the opening ligand gated Cl- channels or
indirectly through other mechanisms the opening of K+ channels
- Cl- flows into the cell
- K+ flows out of the cell
- both increase the negative charge within the cell, hyperpolarizes the soma
- brings membrane potential further away from threshold and so it is harder to
trigger an action potential therefore inhibitory
- an ipsp on the dendrite will have less effect due to current loss than in
ipsp in the soma.
Major ligand gated ion channels and their neurotransmitters in the CNS
Glutamate
- amino acid
- most common excitatory neurotransmitters in central nervous system
- neurotransmitter of NMJ in invertebrates (locust, giant axon of squid)
Glutamate receptor - at least 3 different ligand gated ion channel
receptors for glutamate - all generate epsps as Na+ is the dominant ion that
flows after the channel is open
GABA
- ![]() - aminobutyric
acid
- aminobutyric
acid
- major inhibitory neurotransmitter in the brain
- in some areas of cortex 1 in 5 neurons are GABAergic
GABA receptors
- again many different types of receptors
- the more common GABA receptors are Cl- channels
- usually inhibitory causes an inhibitory postsynaptic potential (IPSP)
- reversal potential is the same as ECl- usually around - 70 mV
Glycine
- simplest amino acid
- major inhibitory neurotransmitter in the brainstem and spinal cord
Glycine Receptor
- major receptor is a Cl- channel
- inhibitory
- like GABA receptor in that usually causes IPSPs
- blocked by strychnine (rat poison) which literally causes convulsions and
death as now the motorneurons are not inhibited and the muscles contract
without control
Remember: Passive current flow in dendrites and cell bodies
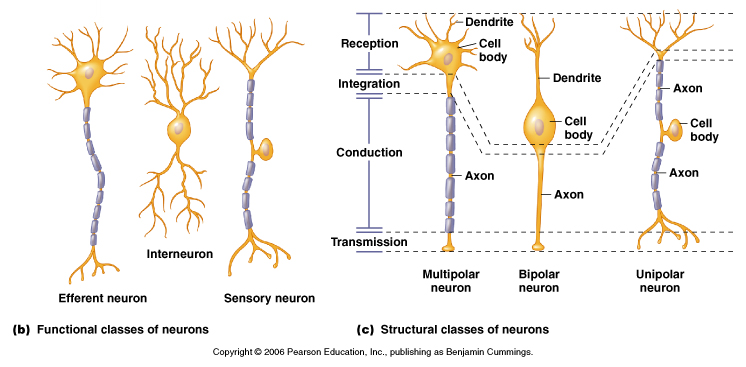
Moyes and Schulte, Figure 5.18 Variation in neuron structure and function.
- dendrites extend 0.5 to 1 mm in all directions from soma and receive
signals from a large area
- 80-90% of all presynaptic terminals terminate on dendrites
- most can't produce action potentials (too few or no Na+ channels)
- transmit current by passive spread down dendrites to the soma
- therefore the membrane potential decreases as move along dendrite due to
current loss thanks to our friends ri, rm and cm
- because dendrites have no voltage gated Na+ channels and cell bodies have
little or no voltage-gated Na+ channels current flow is solely dependent on
the Cable Properties of the dendrites and soma
1) loss of current across membrane (leaky membranes)
- loss of current across membrane results in membrane potential dropping with
distance
- dependent on the internal resistance (ri) and the membrane resistance (rm)
- the length or space constant describes this property
![]()
- ![]() is the distance where Vm is =
0.37Vo
is the distance where Vm is =
0.37Vo
or the distance where the magnitude of the depolarization falls to 1/e of the
initial depolarization. In the figure below the length contant of the small
unmyelintated axon is 0.5 mm (black) and of the large myelinated axon (blue)
is 1.6 mm
- ![]() is dependent on the internal
and membrane resistance
is dependent on the internal
and membrane resistance
= square root of (rm/ri)
- if the membrane resistance is large then the longer the impulse will
travel
- if the internal resistance is large then the shorter the impulse will travel
- length contants also are applicable to dendrites
2) loss of current (charge) due to capacitance properties of the
membrane
- cell membrane acts as a capacitor
- 2 conducting sheets separated by an insulating material
- the closer the sheets the better the capacitor
- lipid bilayer is 7 nm thick therefore = excellent capacitor
- it takes time and current (charge) to charge the membrane capacitor
- as current drops over the length of the nerve takes longer and longer to
charge the capacitor
- the time constant describes this effect
- ![]() is the time it takes to
reach 63% of the final voltage (msec)
is the time it takes to
reach 63% of the final voltage (msec)
- ![]() = Rm x Cm
= Rm x Cm
- the smaller the capacitance properties the less the current loss and the
faster the nerve impulse travels
- the large the capacitance properties the more current loss and the slower
the nerve impulse
- time constants range from 1 to 20 msec.
2) loss of current due to capacitance properties of the membrane
- cell membrane acts as a capacitor
- the large the capacitance properties the more current loss and the slower
the impulse
Motor neuron as a model:
We will concentrate on the motor neuron for the discussion of summation
below. The motorneuron will integrate thousands of different inputs to
generate the signal to either fire an action potential or not fire an action
potential.
The inhibition of the motor neuron is just as important as the excitation. In
vertebrates in particular whether a muscle fiber contracts is controlled at
the level of the motor neuron. Therefore it is essential to be able to inhibit
a motorneuron to ensure that muscle fibers do not contract at the wrong time
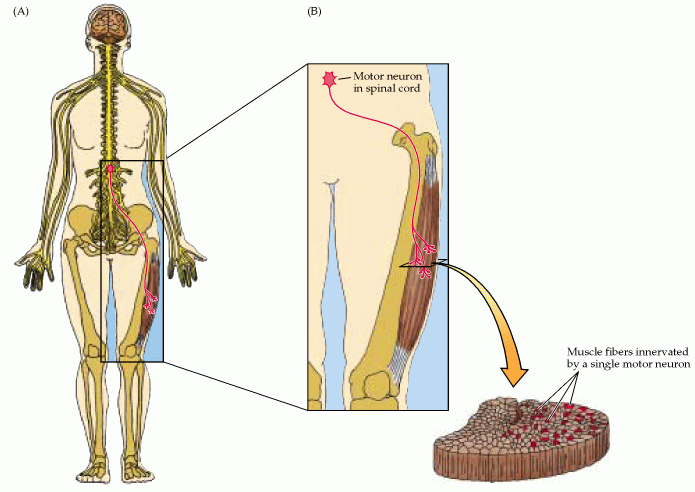
Figure 16.4. The motor unit. (A) Diagram showing a lower motor neuron in the spinal cord and the course of its axon to the muscle. (B) Each motor neuron synapses with multiple muscle fibers. The motor neuron and the fibers it contacts defines the motor unit. Cross section through the muscle shows the distribution of muscle fibers (red dots) contacted by the motor neuron.
Summation:
The postsynaptic effects of most synapses in the brain are not as large as
those at the neuromuscular junction. In the CNS the postsynaptic potentials
are usually far below the threshold for generating postsynaptic action
potentials
Neurons in the central nervous system are typically innervated by thousands of
synapses, and the postsynaptic potentials produced by each active synapse can
summate together (in space and in time) to bring the membrane to threshold for
firing an action potential.
The motor neuron in the example below literally have thousands of excitatory
and inhibitory synapses that spread across both the cell bodies and the
dendrites.
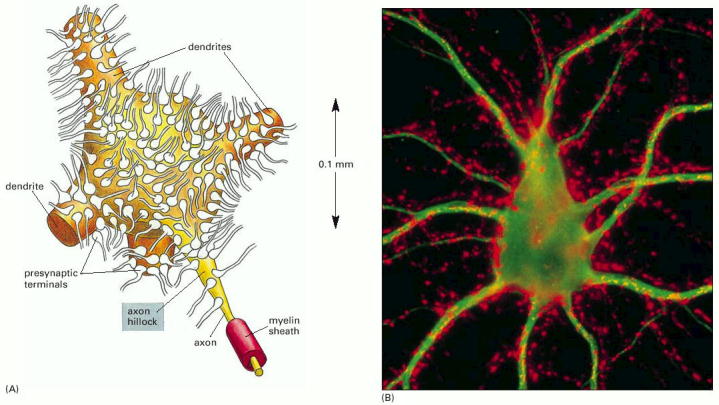
Figure 11-38, Alberts (Molecular Biology of the Cell) 4th edition. A motor neuron cell body in the spinal cord.
(A) Many thousands of nerve terminals synapse on the cell body and dendrites. These deliver signals from other parts of the organism to control the firing of action potentials along the single axon of this large cell. (B) Micrograph showing a nerve cell body and its dendrites stained with a fluorescent antibody that recognizes a cytoskeletal protein (green). Thousands of axon terminals (red) from other nerve cells (not visible) make synapses on the cell body and dendrites; they are stained with a fluorescent antibody that recognizes a protein in synaptic vesicles.
- summation of many inputs from different pre-synaptic cells
- for example an excitatory synapse at a motorneuron in the spinal cord
receives thousands of inputs to its dendrites and cell body
- in the CNS one glutamate synapse usually creates an epsp of ~1mV
- during excitation in a neuronal pool in the nervous system many terminals
are stimulated and the effects can add up together or summate
- soma has a low internal resistance therefore an increase in the membrane
potential in one part affects the whole soma
Figure 21-34, Lodish 4th edition. Figure 7-48 Lodish 5th edition. Summation of multiple epsps to bring the membrane potential to threshold for an action potential.
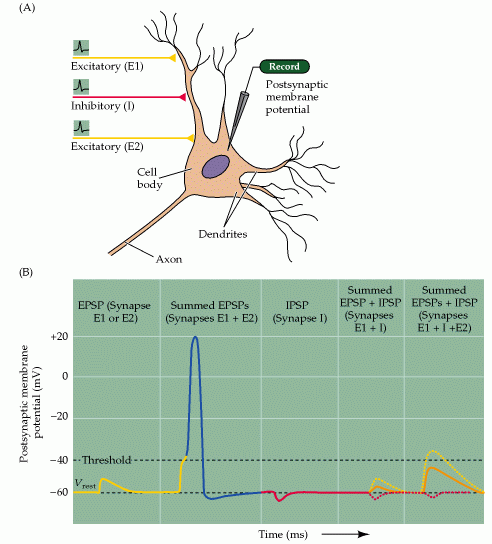
Figure 7.7, Purves (Neuroscience) 2nd edition. Summation of postsynaptic potentials.
(A) A microelectrode records the postsynaptic potentials produced by the activity of two excitatory synapses (E1 and E2) and an inhibitory synapse (I). (B) Electrical responses to synaptic activation. Stimulating either excitatory synapse (E1 or E2) produces a subthreshold EPSP, whereas stimulating both synapses at the same time (E1 + E2) produces a suprathreshold EPSP that evokes a postsynaptic action potential (shown in blue). Activation of the inhibitory synapse alone (I) results in a hyperpolarizing IPSP. Summing this IPSP (dashed red line) with the EPSP (dashed yellow line) produced by one excitatory synapse (E1 + I) reduces the amplitude of the EPSP (orange line), while summing it with the suprathreshold EPSP produced by activating synapses E1 and E2 keeps the postsynaptic neuron below threshold, so that no action potential is evoked.
Neurons that don't generate action potentials
- some cells lack channels to generate action potentials
- therefore all interactions between synaptic input and output sites occurs by
passive spread of current