Structure of the Heart
A human heart is a specialized muscular organ which beats over 2 billion times and pumps over 100 million gallons of blood over the course of the average human lifetime.
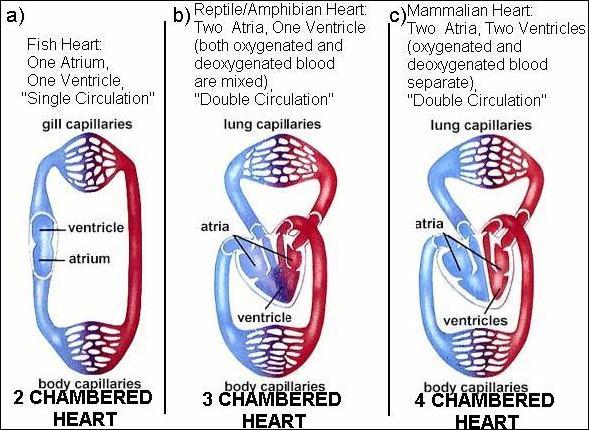
Figure from Campbell's "Biology" 4th edition pg. 822.
Fish have 2 chambers, one atrium and one ventricle. Fish hearts draw in deoxygenated blood in the atrium, and pump it out through a ventricle. Blood enters the heart, gets pumped through the gills and the oxygenated blood goes out to the body.
Amphibians and reptiles have 3 chambers: 2 atria and a ventricle. (Crocodiles are the exception, as they have 4 chambers (2 atria, 2 ventricles). 3 chambered hearts have a complex circuit (blood from heart to lung and back to heart) and must be controlled so the blood travels from the heart to the lungs to become oxygenated and then pumped back the heart and out to the body. In the 3 chambered heart, a single ventricle pumps both out of the heart, and there is some mixing between oxygenated and deoxygenated blood.
The bird and mammalian heart has four chambers:
Atria. The top two chambers that receive blood from the body or lungs.
Ventricles. The bottom two chambers. The right ventricle pumps blood to the
lungs to pick up oxygen, The left ventricle pumps blood to the rest of the
body and is the strongest chamber.
There are 4 steps involved with blood entering the heart: 1) oxygen poor blood
enters the first atrium. 2) oxygen poor blood is fed to the first ventricle,
which pumps it out to the lungs. 3) Oxygen rich blood is pumped back into the
second atria. 4) Oxygen rich blood is pumped by the second ventricle out of
the heart and back into the body.
The 4 chambered heart keeps oxygenated blood completely separate from
de-oxygenated blood, and allows the blood leaving the heart to have far more
oxygen than it would otherwise.
The insect heart is a simple tube. Heart contractions push the blood towards the head but because insects have an open circulatory system, there is no return circuit. The blood moves through the body cavity until it reaches the heart again.
The earthworm has series of five chambers that make up its heart.
Click on the image for a movie of earthworm heart function.
Myogenic versus Neurogenic
For most insects, heart contraction is initiated and regulated predominantly
by external nerves; thus insect hearts are neurogenic.
In contrast, vertebrates, tunicates, and some molluscs have myogenic hearts.
The heart beat is initiated and regulated by specialized groups of muscle
cells.
Cardiac muscle
Cardiac muscle (another type of striated muscle in vertebrates) has many
similar properties to skeletal muscles but there are some important
differences. Hearts of course vary greatly in size, shape and complexity from
animal to animal - ranging from insects with a simple tube that pumps blood or
hemolymph around an open circulatory system to our closed circulatory system
and a four chambered heart. The basic principles that underlie cardiac muscle
cell function though remain pretty much the same.
1) The heart contains pace-maker cells that produce the depolarization and
action potentials to drive cardiac cell contraction. In other words heart
contraction is not neuronally driven but self-driven or myogenically driven.
(Of course there is an exception to every rule and some invertebrates the
pacemaker cells are modified neurons that are attached to the heart). Some
vertebrates hearts are innervated by neurons from the sympathetic and
parasympathetic nervous systems but these neurons act in a modulatory function
only.
2) Each muscle cell is a single cell not multinucleate like skeletal muscle.
Like skeletal muscle cells each cell contains multiple myofibrils and in the
cases of higher vertebrates an extensive sarcoplasmic reticulum and T-tubules.
The SR and T-tubule system is not as extensive in animals with small cardiac
muscle cells (myocytes) such as frogs and in many invertebrates. Therefore
depending on the size of the cardiac muscle cells contraction can depend on
Ca+2 release from the SR and/or Ca+2 influx from external sources outside the
cell. This can be tested as removal of external Ca+2 strongly affects frog
cardiac cell contraction but rat cardiac cells still can contract as they rely
more on release from the SR.
3) Cardiac muscle cells are linked to each other with gap junctions. This
allows an action potentials to rapidly travel from cell to cell and makes the
heart work as a unit. This allows the pacemaker cells, the sinoatrial node
cells, to generate the action potential which is in turn relayed via the gap
junctions throughout the heart to generate contraction through out the heart.
3) There are different types of cardiac muscle cells ranging from the
pacemaker cells in the sinoatrial node to the atrial and ventricular cells
that produce the contraction of the heart chambers. All use the same
mechanisms of excitation-contraction coupling but as we'll see there are
distinct features to the sinoatrial cells that allow them to be pacemakers.
4) The action potential in cardiac cells is quite different from skeletal
muscle and neuronal action potentials in that voltage-gated Ca+2 channels play
a much larger role. See section on cardiac channels and
action potentials
5) The mechanism of triggering the Ca+2 release channel in the sarcoplasmic
reticulum is not the same as in vertebrate skeletal muscle cells. See section
on Ca+2 release channels in the
skeletal muscle lectures to review the differences between the two.
Cardiac muscle channels
Pumps and transporters -
Cardiac cells also have the same types of pumps and transporters as skeletal
muscle cells. In particular they have:
1) Na+/K+ ATPase pump - to establish the electrochemical gradients of Na+ and
K+
2) Ca+2 ATPase pump - uses energy from ATP to remove 2 Ca+2 from the inside to
the outside of the cell or into the sarcoplasmic reticulum to ensure that
internal Ca+2 concentrations remain low (10-7 mM internal). Some
cardiac cells (i.e. lower vertebrates, invertebrates) do not have an extensive
sarcoplasmic reticulum and thus most of the Ca+2 that is used to trigger
contraction is from extracellular sources.
3) Na+/Ca+2 cotransporter - to also remove Ca+2 from the inside of the cell
and uses the energy from the cotransport of 3 Na+ molecules to export 1 Ca+2.
Channels - cardiac muscle cells
share many of the same ion channels as neurons and skeletal muscle cells.
1) leak channels - leak K+ channel
2) voltage-gated Na+ channels - there is a skeletal muscle voltage-gated Na+
channel which properties very much like the neuronal voltage-gated Na+ channel
we have already discussed at length. TTX - sensitive as we saw above. This
channel is of course responsible for the production of the action potential.
Once opened the influx of Na+ through the channel further depolarizes the
membrane thus opening more Na+ channels and this regenerative cycle continues
until the Na+ channels start to inactivate and the delayed K+ channel begins
to open.
3) voltage-gated K+ channel - the delayed rectifier K+ channel which we have
already seen in neurons with exactly the same properties. In other words has a
high threshold and needs a strong depolarization to open and works to bring
the membrane back to resting potentials.
4) voltage gated Ca+2 channels - in cardiac cells the Ca+2 channel plays a
much greater role during the action potential. These channels are the high
threshold Ca+2 channels, called L channel or DHP (dihydropyridine) channel).
The cardiac DHP channel is very similar to the skeletal muscle DHP Ca+2
channel and are found concentrated in the T-tubules in those cardiac cells
with extensive T-tubules and SR.
Action potential in ventricular (and atrial) cardiac cells
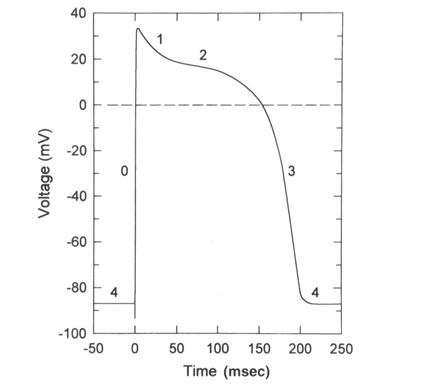
From "Cell Physiology Source Book"
The above diagram is an action potential recorded in the ventricular
cardiac cell of the heart.
4) Resting potentials in these cells is set by a large K+ permeability due to
a combintation of the leak K+ channel and a voltage-gated K+ channel (called
the inward rectifier K+ channel) that is open at rest. This means that rest is
very close to EK+.
0) The rising phase of the action potential is set by the cardiac
voltage-gated Na+ channel which has properties very similar to the neuronal
and skeletal muscle voltage-gated Na+ channels.
1-2) As the voltage-gated Na+ channels produce the rising phase and then start
to inactivate two channels will now be opening, the relayed rectifier K+
channel and the voltage-gated Ca+2 channel (L or DHP channel). There are many
Ca+2 channels in these cells and thus this channel dominates the membrane
potential producing a long plateau of depolarization. This plateau is a
balance between the open Ca+2 channels and the open K+ channels. Remember the
Ca+2 channel only slowly inactivates and thus this plateau can persist for
100-200 msec.
3) Finally the voltage-gated Ca+2 channel inactive and the voltage-gated K+
channels will now dominate and the membrane potential will repolarize to rest
(EK+ in these cells). Then the voltage-gated K+ channels will close, the
voltage-gated Na+ channels will switch from the inactive to the closed state
and the membrane is set back at 4) ready to fire again.
The long Ca+2 plateau allows Ca+2 inside the cell to elevate enough to generate contraction in the case of those cardiac cells that rely on external Ca+2 sources.
Action potential in sinoatrial cardiac cells
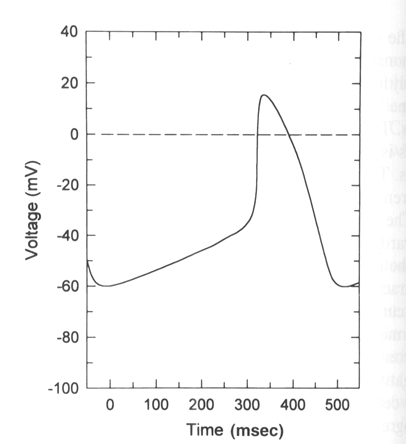
From "Cell Physiology Source Book"
Sinoatrical cells have the ability to spontaneously fire action potentials in a repeated fashion without any external influence. These cells are the pacemaker cells of the heart and once an action potential fires in these cells it is propagated via gap junctions to other regions of the heart first to the atrial cells and then eventually making it to the ventricular cells.
The generation of the action potential in these cells is very similar to
the ventricular cardiac cells with a few major exceptions.
1) These cells do not have a stable rest. There is a spontaneous slow
depolarization that brings the membrane from -60 mV to threshold for the
action potential (about -40 mV).
2) The action potential is driven by the the voltage-gated Ca+2 channel in
most SA cells. The rising phase is due the opening of the voltage-gated Ca+2
channel (L or DHP channel again) and thus is slower than in other excitable
cells.
3) As the Ca+2 channel inactivates the membrane is repolarized by the delayed
rectifier Ka+ channel as in other excitable cells.
4) What makes these cells then spontaneously depolarize once the delayed K+
channel has closed is the presence of an ion channel that is activated by
hyperpolarization. In other words a channel that opens when the membrane
becomes repolarized and allows Na+ to flow into the cell. (Called the funny
channel in some literature). Therefore the Na+ influx will depolarize the
membrane to open the voltage-gated Ca+2 channels and at the same time close
the funny channel.
The funny channel: This unusual cation channel is activated by hyperpolarization. As the membrane repolarizes after the action potential the threshold for opening of the funny channel is reached at about -50 mV. The channel opens and allows Na+ to perferentially flow into the cell. The funny channel is also called the HCN channel or hyperpolarization, cyclic nucleotide gated ion channel. As we will see cAMP can have dramatic influences on this channel and shift its threshold of activation from -50 mV to -40 mV. The funny channel actually looks very much like a voltage-gated K+ channel but has differences in its pore to allow Na+ influx and in the voltage sensing/opening mechanism.
Putting it all together to get a heart beat
The excitatory and electrical conduction system of the heart is responsible for the contraction and relaxation of the heart muscle
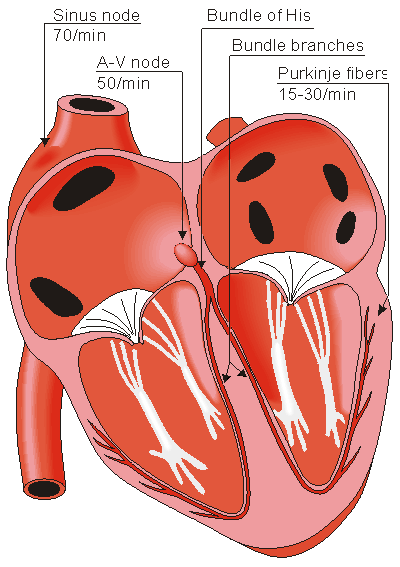
Human heart
Located in the right atrium at the superior vena cava is the sinus node (sinoatrial or SA node) which consists of specialized muscle cells. The SA nodal cells are self-excitatory, pacemaker cells. They generate an action potential at the rate of about 70 per minute in humans (your heart beat). From the sinus node, activation propagates throughout the atria, but cannot propagate directly across the boundary between atria and ventricles, as noted above. This boundary serves to ensure a delay between the activation of the atria and the ventricles. In other words the impulse is delayed at this point to allow complete emptying of the atria before the ventricles contract.
The atrioventricular node (AV node) is located at the boundary between the atria and ventricles. In a normal heart, the AV node provides the only conducting path from the atria to the ventricles. Propagation from the AV node to the ventricles is provided by a specialized muscle cells called the bundle of His conduct the signal system. Further down the bundle separates into two bundle branches which travel along each side of the septum, constituting the right and left bundle branches. Even more distally the bundles split into Purkinje fibers that branch and contact the inner sides of the ventricular walls. From the inner side of the ventricular wall, theese activation sites cause the formation of a wave of depolarization which propagates through gap junctions between the ventricular cells toward the outer wall. After each ventricular muscle region has depolarized, repolarization occurs.
Electrocardiogram - ECG
The different potential generated in the heart can be measure using an electrocardiogram. An Electrocardiogram is a recording of the electric potentials being generated during heart activity. The potentials ("waves") are registered by electrodes placed on certain parts of the body and measure changes in potential (mV). Typically twelve electrodes are used placed in specific regions on the body.
Nerdy Note: In 1924 Willem Einthoven was awarded the Nobel prize in Physiology or Medicine "...for his discovery of the mechanism of the Electrocardiogram."
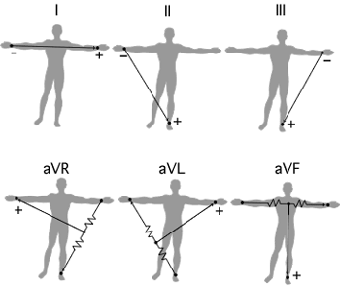
The Leads and Einthoven's Triangle
The electrodes are typically twelve in number. The stretch between two limb (arm or leg) electrodes is called a Lead. Einthoven named the Leads between the three limb electrodes "Standard Lead I, II and III" referring to the two arm electrodes and the left leg electrode. He studied the relationship between these electrodes, forming a triangle where the heart electrically constitutes the null point. The relationship between the Standard Leads is called Einthoven's Triangle. Einthoven's Triangle is used when determining the electrical axis of the heart.
From Nobel Prize ECG Education page
An ECG curve reflects the perspective of the electrode recording it. The standard one seen below is from Standard Lead I, II and III. An ECG curve has different characteristics depending on the location of the electrode recording it. A negative deflection indicates that the recorded wave has traveled away from the electrode and a positive deflection means it has traveled towards it.
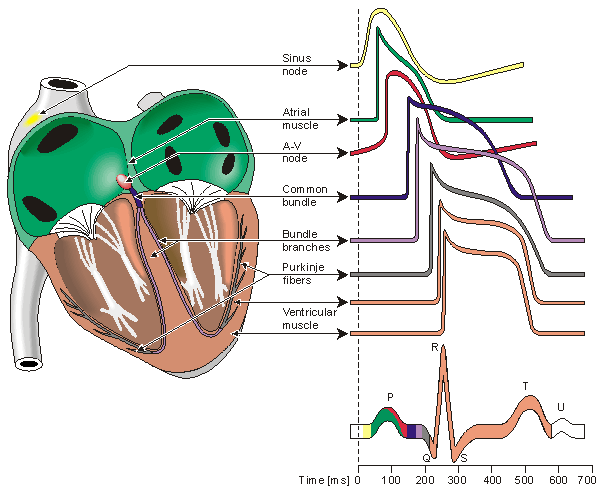
Human heart and different potentials
P wave - an impulse is generated at the sinoatrial node and spreads across
both atria, causing them to contract.
delay - The Fibro-fatty atrioventricular groove insulates the ventricles from
the atrial impulse. The AV node is the only normal gateway of conduction to
the ventricles.
QRS wave - The impulse travels down the AV bundle and it's branches and
reaches the Purkinje fibers. The ventricles are stimulated to contract.
T wave - correlates with repolarization of the ventricles.
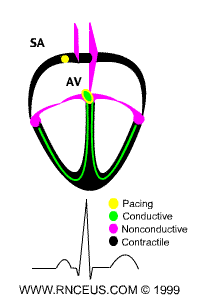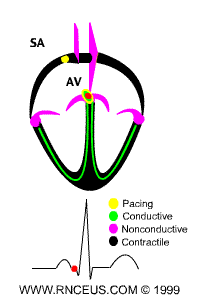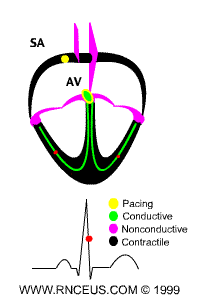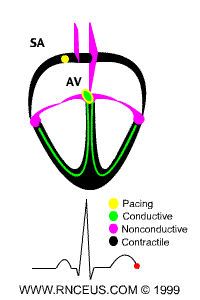
ECG of human heart and regions of contraction
Modification of cardiac muscle function: Increased heart rate
Release of neurotransmitters from the autonomic nervous systems can increase or decrease heart contraction by directly affecting the funny channel or other ion channels in the membrane. Think about the physiological responses associated with adrenalin (epinephrine).
Figure 21-6, Lodish 4th edition. A highly schematic diagram of the vertebrate nervous system.
The central nervous system (CNS) comprises the brain and spinal cord. It receives direct sensory input from the eyes, nose, tongue, and ears. The peripheral nervous system (PNS) comprises three sets of neurons: (1) somatic and visceral sensory neurons, which relay information to the CNS from receptors in somatic and internal organs; (2) somatic motor neurons, which innervate voluntary skeletal muscles; and (3) autonomic motor neurons, which innervate the heart, the smooth involuntary muscles such as those surrounding the stomach and intestine, and glands such as the liver and pancreas. The sympathetic and parasympathetic autonomic motor neurons frequently cause opposite effects on internal organs. The cell bodies of somatic motor neurons are within the CNS; those of somatic sensory neurons and of autonomic motor neurons are in ganglia adjacent to the CNS.
Epinephrine and norepinephrine: released from the sympathetic nervous system. Epinephrine and norepinephrine is synthesized and released into the blood by the adrenal medulla, an endocrine organ. Epinephrine and the related norepinephrine are all synthesized from tyrosine and contain the catechol moiety; hence they are referred to as catecholamines. Nerves that synthesize and use epinephrine or norepinephrine are termed adrenergic.
Adrenergic receptors: bind epinephrine and norepinephrine. Because different receptors are linked to different G proteins, the activation leads to different signal transduction cascades. For instance, binding of norepinephrine to beta-adrenergic receptors on nerve cells causes activation of Gs and an increase in cAMP synthesis. Other neuronal adrenergic receptors activate Gi, Go, or other types of G proteins, resulting in a decrease in cAMP levels or increases in the levels of other intracellular second messengers, such as cGMP, inositol 1,4,5-trisphosphate (IP3), diacylglycerol, and arachidonic acid. Some second messengers, such as cGMP and IP3, act to directly open or close ion channels in neurons; IP3, for example, opens Ca2+ channels in the membrane of the endoplasmic reticulum, causing an increase in cytosolic Ca2+. Other second messengers have a more indirect effect on ion channel.
In sinoatrial cells: norepinephrine binds to the b-adrenergic receptor which is a G protein associated membrane receptor. This triggers a signal transduction cascade outlined below that activates the G protein (Gs - stimulates) that activates adenylate cyclase to produce cAMP.
Beta-blockers: Drugs which are used to slow heart contractions in the treatment of cardiac arrhythmia and angina, are beta1-adrenergic receptor antagonists. They bind the beta1-adrenergic receptor to block the receptor and thus slow heart contraction. Cardiac muscle cells possess beta1 adenergic receptors. The beta blockers usually have little effect on beta-adrenergic receptors on other cell types. The smooth muscle cells lining the bronchial passages possess b2 receptors, which mediate relaxation by binding catecholamines with the rank order of affinities isoproterenol >> epinephrine = norepinephrine. Agonists selective for b2 receptors, such as terbutaline, are used in the treatment of asthma because they specifically mediate opening of the bronchioles, the small airways in the lungs.
Modulating the funny channel
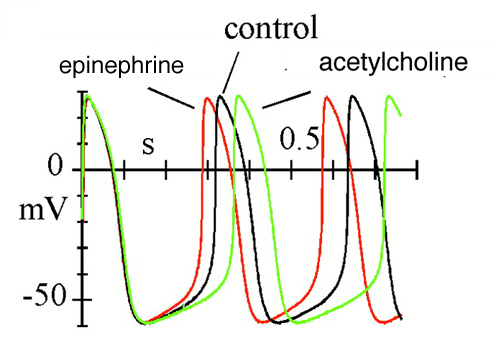
Effects of autonomic agonists on spontaneous activity and hyperpolarization-activated current (If) in cardiac sinoatrial node (SAN) myocytes from the rabbit.
Spontaneous action potentials recorded in control conditions and in the presence of either isoprenaline (Iso) or acetylcholine (ACh).
News Physiol Sci (2002) 17: 32-37.
Through G protein coupled receptors various hormones/neurotransmitters or drugs can increase or decrease the heart rate by simply increasing or decreasing the ability of the funny channel to open.
|
|
Figure 20-16, Lodish 4th edition. Activation of adenylyl cyclase
following binding of an appropriate hormone (e.g., epinephrine, glucagon)
to a Gs protein coupled receptor. Following ligand binding to the
receptor, the Gs protein relays the hormone signal to the effector
protein, in this case adenylyl cyclase. Gs cycles between an inactive
form with bound GDP and an active form with bound GTP. Dissociation of
the active form yields the Gsalpha ∑ GTP complex, which directly
activates adenylyl cyclase. Activation is short-lived because GTP is
rapidly hydrolyzed (step 5). This terminates the hormone signal and
leads to reassembly of the inactive Gs ∑ GDP form, returning the system
to the resting state. Binding of another hormone molecule causes
repetition of the cycle. Both the Ggamma and Gsalpha subunits are linked
to the membrane by covalent attachment to lipids. Binding of the
activated receptor to Gsalpha promotes dissociation of GDP and its
replacement with GTP.
|
The increase in cAMP physically binds to the funny channel and makes the channel open more easily. In other words the threshold for opening shifts from around -50 mV to around -40 mV. Therefore the funny channel will open sooner during the repolarization stage of the sinoatrial action potential and a second action potential will be triggered sooner. This means that a second wave of action potential and thus contraction will travel through the heart sooner ie. a faster heart rate.
Modulation of Ca+2 channel
Epinephrine also causes an increase in cAMP that stimulates PKA (protein kinase A) which in turn phosphorylates the voltage-gated Ca+2 channel (L channel). This phosphorylation results in a protein conformational change that enchances the channels activity. This new conformation of Ca+2 channel opens more readily (i.e. less time between action potentials) and opens for longer (i.e. more Ca+2 flow into the cell = greater [Ca+2] intracellular = greater contraction).
[Note: Epinephrine also stimulates glycogen breakdown in skeletal muscles. Epinephrine through binding to it's G protein associated receptor results in the G protein stimulation of an enzyme glycogen phosphorylase kinse. As its name implies glycogen phosphorylase kinase phosphorylates glycogen phosphorylase to activate this enzyme. As you all remember glycogen phosphorylase takes glycogen and breaks it down into glucose1-phosophate to initiate glycolysis. Glycogen phosphorylase kinase can be stimulated by two mechanisms G protein and increase in Ca+2 internally and in this way during periods of concentrated activity the glycogen energy stores of muscles can be mobilized.]
Caffeine: blocks the activty of phosphodiesterases. Phosphodiesterases break down cyclic nucleotides. Therefore in the presence of caffeine cAMP levels remain elevated and thus the funny channel continues to open more readily. Therefore the sinoatrial action potential fires more frequently and heart rate is increased.
[Note:Caffeine also affects the Ca+2 release channel or ryanodine receptor such that more Ca+2 is released through the channel. Therefore heart contractions are stronger in the presence of caffeine as well.]
Modification of cardiac muscle function: Decreased heart rate
Acetylcholine: released from parasympathetic nervous system.

Amanita muscaria. Source of plant steroid muscarine
Muscarinic acetylcholine receptor: a G protein associated receptor. The G protein activated in this case is a Gi asubunit that inhibits adenylate cyclase.
Modulating the funny channel
Figure 20-18, Lodish 4th edtion. Epinephrine activates adenlyl cyclase while acetylcholine inhibits.
Acetylcholine works to block any rise in cAMP and reduces cAMP levels in the cell. Therefore the funny channel will now not open so readily and the slow depolarziation of the membrane will occur later thus resulting in a longer time to generate a second action potential.

Effects of autonomic agonists on spontaneous activity and hyperpolarization-activated current (If) in cardiac sinoatrial node (SAN) myocytes from the rabbit.
Spontaneous action potentials recorded in control conditions and in the presence of either isoprenaline (Iso) or acetylcholine (ACh).
News Physiol Sci (2002) 17: 32-37.
Modulating the voltage-gated K+ channels
Figure 21-41, Lodish 4th edition. Acetylcholine-induced opening of K+ channels in the heart muscle plasma membrane.
Binding of acetylcholine by muscarinic acetylcholine receptors triggers activation of a transducing G protein by catalyzing exchange of GTP for GDP on the alpha subunit. The released beta/gamma subunit then binds to and opens a K+ channel. The increase in K+ permeability hyperpolarizes the membrane, which reduces the frequency of heart muscle contraction. Activation is terminated when the GTP bound alpha subunit is hydrolyzed to GDP and Galpha ∑ GDP recombines with Gbeta/gamma.
Acetylcholine also works through the muscarinic receptor to stimulate the opening of a K+ channel. The muscarinic receptor stimulates the release of the G protein beta/gamma that directly activates a K+ channel. The opening of the channel suppresses the excitability of the membrane and thus suppresses muscle and heart contraction
Figure 21-32, Lodish 4th edition. Inhibitory responses in postsynaptic cells stimulated by acetylcholine.
(b) Application of acetylcholine (or muscarine) to frog heart muscle produces, after a lag period of about 40 ms (not visible in graph), a hyperpolarization of 2 ≠ 3 mV, which lasts several seconds. These cells contain muscarinic acetylcholine receptors, which are coupled via a G protein to K+ channels. Activation of the receptor leads to channel opening.
Digitalis
Specifically Inhibits the Na+/K+ Pump by Blocking Its Dephosphorylation
Digitalis and ouabain are steroids derived from plants that are inhibitors of
the Na+-K+ pump. These are known as cardiotonic steroids because of their
strong effects on the heart. They inhibit the dephosphorylation of the
phosphorylated form of the ATPase when applied on the extracellular face of
the membrane.

Digitalis, steroids from the dried leaf of the foxglove plant (Digitalis purpurea)
Digitalis increases the force of contraction of heart muscle and is a drug used the treatment of congestive heart failure. Inhibition of the Na+/K+ pump by digitalis leads to a higher level of Na+ inside the cell. This results in a less favourable DeltaG for Na+ transport. The diminished Na+ gradient results in slower extrusion of Ca2+ by the sodiumócalcium exchanger.
In cardiac muscle cells, a Na+/Ca2+ antiporter, is the major way the cell maintains a low concentration of Ca2+ in the cytosol. The movement of three Na+ ions is required to power the export of one Ca2+ ion against the greater than 10,000-fold concentration gradient between the cell interior and cell exterior.
The increase in the intracellular level of Ca2+ enhances the contractility of cardiac muscle (remember contraction intensity is directly related to myoplasmic Ca+2 levels).